Maintenance of a Somatotopic Cortical Map in the Face of Diminishing Thalamocortical Inputs (Plasticity͞divergence͞cortical Magnification)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

TRPV1-Like Immunoreactivity in the Human Locus K, a Distinct Subregion of the Cuneate Nucleus
cells Article TRPV1-Like Immunoreactivity in the Human Locus K, a Distinct Subregion of the Cuneate Nucleus Marina Del Fiacco 1 ID , Maria Pina Serra 1 ID , Marianna Boi 1, Laura Poddighe 1, Roberto Demontis 2, Antonio Carai 2 and Marina Quartu 1,* 1 Department of Biomedical Sciences, University of Cagliari, Cittadella Universitaria di Monserrato, 09042 Monserrato (CA), Italy; marina.delfi[email protected] (M.D.F.); [email protected] (M.P.S.); [email protected] (M.B.); [email protected] (L.P.) 2 Department of Medical Sciences and Public Health, University of Cagliari, Cittadella Universitaria di Monserrato, 09042 Monserrato (CA), Italy; [email protected] (R.D.); [email protected] (A.C.) * Correspondence: [email protected]; Tel.: +39-070-675-4084 Received: 29 April 2018; Accepted: 5 July 2018; Published: 8 July 2018 Abstract: The presence of transient receptor potential vanilloid type-1 receptor (TRPV1)-like immunoreactivity (LI), in the form of nerve fibres and terminals, is shown in a set of discrete gray matter subregions placed in the territory of the human cuneate nucleus. We showed previously that those subregions share neurochemical and structural features with the protopathic nuclei and, after the ancient name of our town, collectively call them Locus Karalis, and briefly Locus K. TRPV1-LI in the Locus K is codistributed, though not perfectly overlapped, with that of the neuropeptides calcitonin gene-related peptide and substance P, the topography of the elements immunoreactive to the three markers, in relation to each other, reflecting that previously described in the caudal spinal trigeminal nucleus. Myelin stainings show that myelinated fibres, abundant in the cuneate, gracile and trigeminal magnocellular nuclei, are scarce in the Locus K as in the trigeminal substantia gelatinosa. -

Modality-Based Organization of Ascending Somatosensory Axons in the Direct Dorsal Column Pathway
The Journal of Neuroscience, November 6, 2013 • 33(45):17691–17709 • 17691 Cellular/Molecular Modality-Based Organization of Ascending Somatosensory Axons in the Direct Dorsal Column Pathway Jingwen Niu,1 Long Ding,1 Jian J. Li,2 Hyukmin Kim,3 Jiakun Liu,1 Haipeng Li,1,4 Andrew Moberly,1 Tudor C. Badea,5 Ian D. Duncan,6 Young-Jin Son,3 Steven S. Scherer,2 and Wenqin Luo1 1Department of Neuroscience and 2Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104, 3Shriners Hospital Pediatric Research Center and Department of Anatomy and Cell Biology, Temple University School of Medicine, Philadelphia, Pennsylvania 19140, 4Department of Neurology, the First People’s Hospital of Chenzhou, Chenzhou, Hunan, China, 5Retinal Circuit Development & Genetics Unit, National Eye Institute, Bethesda, Maryland 20892, and 6Department of Medical Sciences, School of Veterinary Medicine, University of Wisconsin, Madison, Wisconsin 53706 The long-standing doctrine regarding the functional organization of the direct dorsal column (DDC) pathway is the “somatotopic map” model, which suggests that somatosensory afferents are primarily organized by receptive field instead of modality. Using modality- specific genetic tracing, here we show that ascending mechanosensory and proprioceptive axons, two main types of the DDC afferents, are largely segregated into a medial–lateral pattern in the mouse dorsal column and medulla. In addition, we found that this modality-based organization is likely to be conserved in other mammalian species, including human. Furthermore, we identified key morphological differences between these two types of afferents, which explains how modality segregation is formed and why a rough “somatotopic map” was previously detected. -

Restoring Somatosensation: Advantages and Current Limitations of Targeting the Brainstem Dorsal Column Nuclei Complex
fnins-14-00156 February 26, 2020 Time: 18:8 # 1 PERSPECTIVE published: 28 February 2020 doi: 10.3389/fnins.2020.00156 Restoring Somatosensation: Advantages and Current Limitations of Targeting the Brainstem Dorsal Column Nuclei Complex Alastair J. Loutit and Jason R. Potas* School of Medical Sciences, UNSW Sydney, Sydney, NSW, Australia Current neural prostheses can restore limb movement to tetraplegic patients by translating brain signals coding movements to control a variety of actuators. Fast and accurate somatosensory feedback is essential for normal movement, particularly dexterous tasks, but is currently lacking in motor neural prostheses. Attempts to restore somatosensory feedback have largely focused on cortical stimulation which, thus far, have succeeded in eliciting minimal naturalistic sensations. Yet, a question that deserves more attention is whether the cortex is the best place to activate the central nervous system to restore somatosensation. Here, we propose that the brainstem dorsal column nuclei are an ideal alternative target to restore somatosensation. We review some of Edited by: Alejandro Barriga-Rivera, the recent literature investigating the dorsal column nuclei functional organization and The University of Sydney, Australia neurophysiology and highlight some of the advantages and limitations of the dorsal Reviewed by: column nuclei as a future neural prosthetic target. Recent evidence supports the dorsal Solaiman Shokur, Federal Institute of Technology column nuclei as a potential neural prosthetic target, but also identifies several gaps in in Lausanne, Switzerland our knowledge as well as potential limitations which need to be addressed before such Aneesha Krithika Suresh, a goal can become reality. University of Chicago, United States *Correspondence: Keywords: neural coding, brain-machine interface, neuroprosthesis, cuneate, gracile, tactile, proprioception, Jason R. -

Medial Lemniscal and Spinal Projections to the Macaque Thalamus
The Journal of Neuroscience, May 1994, 14(5): 2485-2502 Medial Lemniscal and Spinal Projections to the Macaque Thalamus: An Electron Microscopic Study of Differing GABAergic Circuitry Serving Thalamic Somatosensory Mechanisms Henry J. Ralston III and Diane Daly Ralston Department of Anatomy, W. M. Keck Foundation Center for Integrative Neuroscience, University of California, San Francisco, California, 94143-0452 The synaptic relationships formed by medial lemniscal (ML) jority of these spinal afferents suggests that the transmis- or spinothalamic tract (STT) axon terminals with neurons of sion of noxious information is probably not subject to GA- the somatosensory ventroposterolateral thalamic nucleus of BAergic modulation by thalamic interneurons, in contrast to the macaque monkey have been examined quantitatively by the GABAergic processing of non-noxious information car- electron microscopy. ML and STT axons were labeled by the ried by the ML afferents. The differences in the GABAergic anterograde axon transport of WGA-HRP following injection circuits of the thalamus that mediate ML and STT afferent of the tracer into the contralateral dorsal column nuclei, or information are believed to underlie differential somatosen- the dorsal horn of the spinal cord, respectively. Thalamic sory processing in the forebrain. We suggest that changes tissue was histochemically reacted for the presence of HRP. in thalamic GABAergic dendritic appendages and GABA re- Serial thin sections were stained with a gold-labeled anti- ceptors following CNS injury may play a role in the genesis body to GABA, to determine which neuronal elements ex- of some central pain states. hibited GABA immunoreactivity (GABA-ir). Serially sec- [Key words: thalamus, somatosensory, monkey, GABA, tioned thalamic structures were recorded in electron medial lemniscus, spinothalamic tract, inhibition, interneu- micrographs and reconstructed in three dimensions by com- ron, pain] puter. -

Somatic Sensory System Objective • to Learn the Functional Organization
Somatic Sensory System Objective • To learn the functional organization of the pathways for touch, limb position sense, and pain and temperature senses • To be able to localize the site of damage to the CNS by evaluating changes in somatic sensory function. NTA Ch. 5 Key Figs: 5-1; 5-7; 5-8; 5-9-5-10 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding Note on NTA Chapters 5-15 are each divided into 2 parts. The first present salient features of the functional organization of the neural system. These sections are short enough for you to manage to read before attending lab. The second part describes the anatomical organization, and is in more detail. You will find it helpful to read the second part after you attend lab. This will serve as a review of material covered with your lab instructor. ************************************************************************************** List of media A-1 Receptors of the Glabrous Skin. This slide illustrates a section through the glabrous skin. Meissner’s corpuscles and Pacinian corpuscles are rapidly adapting mechanoreceptors Merkel’s discs (receptors) are slowly adapting mechanoreceptors. These mechanoreceptors are the distal portions of large diameter primary afferent fibers. In glabrous skin, the free nerve endings, the distal processes of small diameter primary afferent fibers, are nociceptors and thermoreceptors. What somatic sensation do Pacinian corpuscles mediate? What receptors mediate pain? A-6 Upper spinal and brain stem course of the dorsal column-medial lemniscal system. This and the next slide illustrate the three-dimensional configuration of the major ascending somatic sensory systems; do not be concerned with details now. -

Postsynaptic Dorsal Column Pathway of the Rat. III. Distribution of Ascending Afferent Fibers
The Journal of Neuroscience, September 1969, g(9): 31463166 Postsynaptic Dorsal Column Pathway of the Rat. III. Distribution of Ascending Afferent Fibers Kenneth D. Cliffera and Glenn J. Giesler, Jr. Department of Cell Biology and Neuroanatomy, University of Minnesota, Minneapolis, Minnesota 55455 The distribution in the dorsal column nuclei (DCn) of post- Lu et al., 1983; Giesler et al., 1984; Kamogawa and Bennett, synaptic dorsal column (PSDC) fibers was examined in rats 1986). Because these dorsal horn neurons are postsynaptic to following injections of Phaseolus vulgaris leucoagglutinin primary afferent fibers, the term postsynaptic dorsal column (PHA-L) in the spinal cord. Lemniscal neurons in the DCn neurons has been frequently used to describe them. The cells of were retrogradely labeled in the same animals by injecting origin of the postsynaptic dorsal column (PSDC) pathway in the thalamus with Fluoro-Gold. In some experiments, primary cats have been shown to transmit nociceptive information afferent fibers were also labeled by injecting dorsal root (Uddenberg, 1966, 1968b; Petit, 1972; Angaut-Petit, 1975b; Lu ganglia with choleragenoid-conjugated HRP. Injections of et al., 1983; Bennett et al., 1984; Kamogawa and Bennett, 1986). PHA-L into the cervical enlargement labeled many fibers and In addition, nociceptive responses have been recorded in the varicosities throughout most of the ipsilateral cuneate nu- portion of the DCn of cats that receives PSDC input (Dart and cleus. Labeled fibers were also present in the external cu- Gordon, 1973; Angaut-Petit, 1975b). Postsynaptic afferent fi- neate and internal basilar nuclei. Injections of PHA-L into bers appear to terminate exclusively in the rostra1 and ventral thoracic cord labeled fibers and varicosities in the medial regions of the DCn in cats, avoiding the “cell cluster” regions cuneate and lateral gracile nuclei, as well as the external of both nuclei (Rustioni, 1973, 1974). -

The Dorsal Column Nuclei Neuroanatomy Reveals a Complex Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 November 2019 doi:10.20944/preprints201911.0084.v1 The Dorsal Column Nuclei Neuroanatomy Reveals A Complex Sensorimotor Integration and Distribution Hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] Number of words (excluding references, abstract and abbreviations): 14,490 Number of figures: 6 Number of Tables: 1 Number of supplementary figures: 0 Keywords: brainstem sensory nuclei; somatosensation; secondary afferents; posterior column Abstract The dorsal column nuclei (DCN) are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. In this review, we examine the morphology, organisation, and connectivity of the DCN and their associated nuclei, to improve understanding of their sensorimotor functions. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the constituents of the dorsal column nuclei complex (DCN-complex), which includes the gracile, cuneate, external cuneate, X, and Z nuclei, and Bischoff’s nucleus. Finally, we examine and discuss the functional implications of the myriad of DCN-complex projection targets throughout the midbrain, and hindbrain, in addition to their modulatory inputs from the cortex. -

The Somatosensory System, with Emphasis on Structures Important for Pain
BRAIN RESEARCH REVIEWS 55 (2007) 297– 313 available at www.sciencedirect.com www.elsevier.com/locate/brainresrev Review The somatosensory system, with emphasis on structures important for pain William D. Willis Jr. Department of Neuroscience and Cell Biology, University of Texas Medical Branch, 301 University Blvd., Galveston, TX 77555-1069, USA ARTICLE INFO ABSTRACT Article history: Santiago Ramón y Cajal described a number of somatosensory structures, including several Accepted 20 May 2007 associated with pain, in his major work on the Histology of the Nervous System of Man and Available online 12 June 2007 Vertebrates. Our knowledge of such structures has been considerably expanded since Cajal because of the introduction of a number of experimental approaches that were not Keywords: available in his time. For example, Cajal made several drawings of peripheral TRP receptor mechanoreceptors, as well as of bare nerve endings, but later work by others described Functional polarity additional somatosensory receptors and investigated the ultrastructure of bare nerve Lissauer's tract endings. Furthermore, the transducer molecules responsible for responses to nociceptive, Anterograde axonal transport thermal or chemical stimuli are now becoming known, including a series of TRP (transient Dorsal column-medial receptor potential) receptor molecules, such as TRPV1 (the capsaicin receptor). Cajal lemniscus pathway described the development of dorsal root and other sensory ganglion cells and related the disposition of their somata and neurites to his theory of the functional polarity of neurons. He described the entry of both large and small afferent fibers into the spinal cord, including the projections of their collaterals into different parts of the gray matter and into different white matter tracts. -
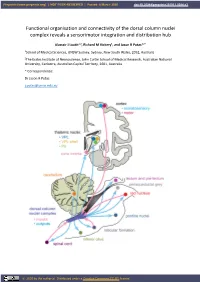
Functional Organisation and Connectivity of the Dorsal Column Nuclei Complex Reveals a Sensorimotor Integration and Distribution Hub
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Functional organisation and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub Alastair J Loutit1,2, Richard M Vickery1, and Jason R Potas1,2 * 1School of Medical Sciences, UNSW Sydney, Sydney, New South Wales, 2052, Australia 2The Eccles Institute of Neuroscience, John Curtin School of Medical Research, Australian National University, Canberra, Australian Capital Territory, 2601, Australia * Correspondence: Dr Jason R Potas [email protected] © 2020 by the author(s). Distributed under a Creative Commons CC BY license. Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 8 March 2020 doi:10.20944/preprints201911.0084.v3 Abstract The dorsal column nuclei complex (DCN-complex) includes the dorsal column nuclei (DCN, referring to the gracile and cuneate nuclei collectively), external cuneate, X, and Z nuclei, and the median accessory nucleus. The DCN are organised by both somatotopy and modality, and have a diverse range of afferent inputs and projection targets. The functional organisation and connectivity of the DCN implicate them in a variety of sensorimotor functions, beyond their commonly accepted role in processing and transmitting somatosensory information to the thalamus, yet this is largely underappreciated in the literature. To consolidate insights into their sensorimotor functions, this review examines the morphology, organisation, and connectivity of the DCN and their associated nuclei. First, we briefly discuss the receptors, afferent fibres, and pathways involved in conveying tactile and proprioceptive information to the DCN. Next, we review the modality and somatotopic arrangements of the remaining constituents of the DCN-complex. -

A Neurotoxicological Perspective by Joseph C
Environmental Health Perspectives Vol. 44, pp. 23-30, 1982 Structure and Function of the Somatosensory System: A Neurotoxicological Perspective by Joseph C. Arezzo*, Herbert H. Schaumburg* and Peter S. Spencer* The somatosensory system comprises those elements of the peripheral nervous system (PNS) and the central nervous system (CNS) subserving the modalities of touch, vibration, tempera- ture, pain and kinesthesia. Specific modalities can be associated with unique peripheral receptors, peripheral axons of stereotyped diameter and specific central projection pathways. Several features of the somatosensory system render regions of it vulnerable to a wide variety of toxicants. The present report highlights these features and, furthermore, suggests that analysis of these regions is invaluable in studying the three most common varieties of toxic neuropathy: toxic distal axonopathy, toxic myelinopathy and toxic sensory neuronopathy. nociceptors. These categories are further subdivided Receptors on the basis of location and rate of adaptation. Mechanoreceptors are sensitive to nondamaging Structure and Function mechanical disturbances of skin or hair. An exam- Since the early nineteenth century, various spe- ple of a slowly adapting position detector is a Type I cialized receptors have been associated with the Iggo corpuscle, featured by a myelinated axon ter- somatosensory system. These receptors, located in minating at the base of a small dome-like elevation skin, hair follicles, joints and muscles, transduce in the skin (Merkel cells). Displacement of the dome mechanical or thermal energy. Minimally, a recep- by as little as 5 ,um can result in a supra-threshold tor includes a peripheral axon terminal of one pri- generator potential within the Merkel cell-axon ter- mary afferent neuron, whose cell body is sited minal complex. -

The Brain in Two Pages
91/10/15 13:55:02 THE_BRAIN_IN_TWO_PAGES 1 NEOCORTEX --muscle length (muscle spindles) 1) excitatory cells (spiny--long range axons) --force exerted by muscle (golgi tendon organs) --pyramidal (many subclasses) 2) dorsal root ganglion cells => spinal cord, dorsal column nuclei --spiny stellate (only in layer 4) 3) spinal cord (dorsal horn) 2) inhibitory cells (no spines--local axons) --spinocervical: spinal cord => lateral cervical nucleus --basket (large) --spinothalamic: spinal cord => posterior nuclei, intralaminar nuclei --chandelier (small, synapse on pyramidal axons’ initial seg) 4) spinal cord (ventral horn) --double bouquet (very small, vertical) --pattern generators --clutch (very small) --motor neurons (synapse on muscles) 3) layers of the cortex 5) dorsal column nuclei --layer 1 (feedback input layer) --cuneate nucleus (hand and upper body) => ventrobasal thalamus --layers 2/3 (feedforward cortical output layer) --gracile nucleus (foot and lower body) => ventrobasal thalamus --layer 4 (feedforward input) 6) principal sensory nucleus of the trigeminal (face) --layer 5 (descending output => striatum, SC, pontine nuc., spinal crd) --also => ventrobasal thalamus --layer 6 (feedback output layer) 7) ventrobasal thalamus (= VPL + VPM) --entire body reprensetation CEREBELLAR CORTEX --projects to cortical areas 3b, 1, and 2 1) input axons (excitatory) 8) intralaminar nuclei (in dorsal thalamus) --mossy fibers from many sources 9) cortical areas list --climbing fibers from inferior olive --3a, 3b, 1, 2, 5, 7b, S-II, Ig, VS 2) inhibitory