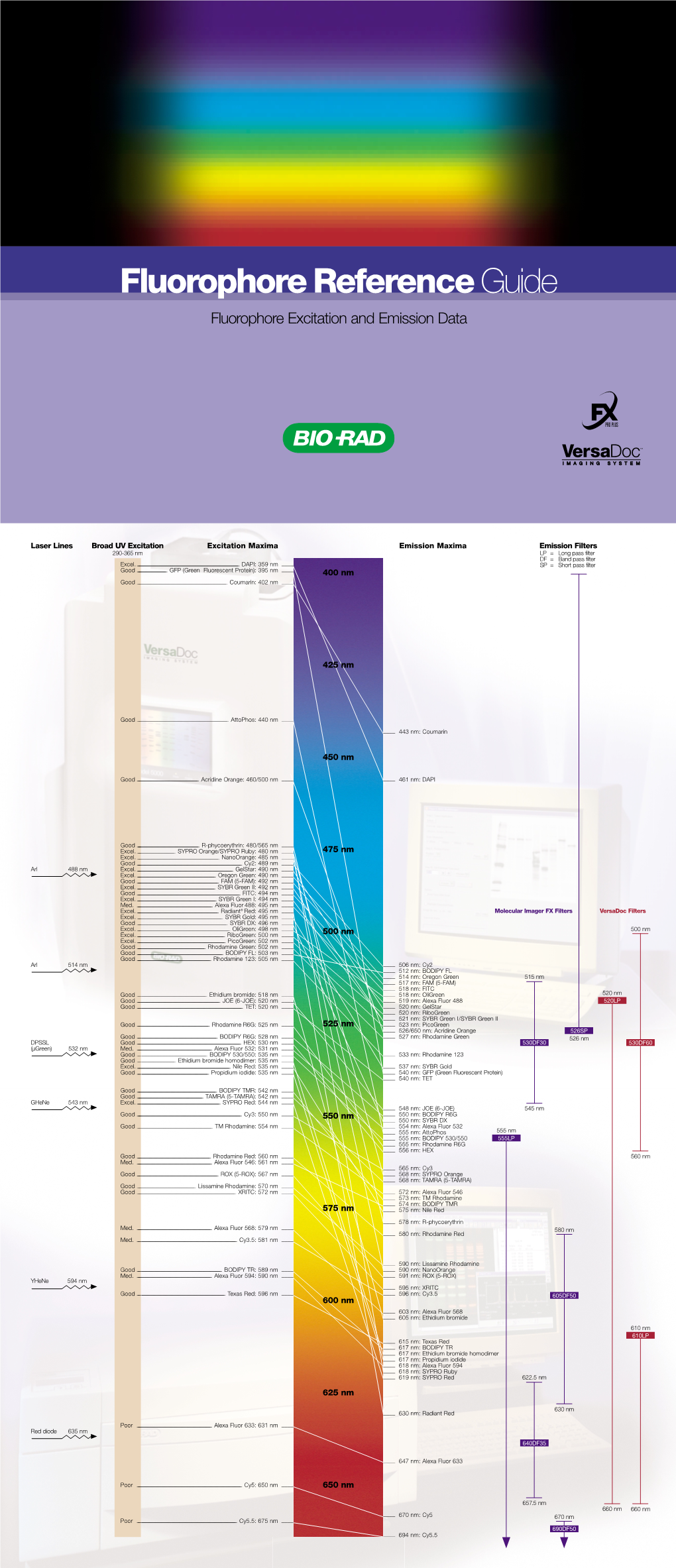
Fluorophore Referenceguide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Contrast to Specific Cancer Genes, Susceptibility Genes As Exemplified
CYP1B1 EXPRESSION, A POTENTIAL RISK FACTOR FOR BREAST CANCER 1 Regine Goth-Goldstein2, Christine A. Erdmann, and Marion Russell Lawrence Berkeley National Laboratory, Environmental Energy Technologies Division, One Cyclotron Road, Berkeley, CA 94720 1 Running Title: CYP1B1 Expression in Breast Tissue Key Words: CYP1B1, CYP1A1, expression, breast, polycyclic aromatic hydrocarbons Footnotes 1 This research was supported by USAMRMC Grant No. DAMD17-98-1-8062 through the U.S. Department of Energy under Contract No.DE-AC03-76SF00098. 2 To whom requests for reprints should be addressed at Lawrence Berkeley National Laboratory, Mail Stop 70-108B, One Cyclotron Road, Berkeley, CA 94720, Phone: (510) 4865897; Fax: 5(10) 4867303; E-mail: [email protected] 3 The abbreviations used are : B[a]P, benzo[a]pyrene; bp, base pair; CYP1A1, cytochrome P4501A1; CYP1B1, cytochrome P4501B1; HMEC, human mammary epithial cells; met, metastasis; ln, lymphnode; PAHs, polycyclic aromatic hydrocarbons; SD, standard deviation. 4Disclaimer: This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor The Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by 2 the United States Government or any agency thereof, or The Regents of the University of California. -

The Green Fluorescent Protein
P1: rpk/plb P2: rpk April 30, 1998 11:6 Annual Reviews AR057-17 Annu. Rev. Biochem. 1998. 67:509–44 Copyright c 1998 by Annual Reviews. All rights reserved THE GREEN FLUORESCENT PROTEIN Roger Y. Tsien Howard Hughes Medical Institute; University of California, San Diego; La Jolla, CA 92093-0647 KEY WORDS: Aequorea, mutants, chromophore, bioluminescence, GFP ABSTRACT In just three years, the green fluorescent protein (GFP) from the jellyfish Aequorea victoria has vaulted from obscurity to become one of the most widely studied and exploited proteins in biochemistry and cell biology. Its amazing ability to generate a highly visible, efficiently emitting internal fluorophore is both intrin- sically fascinating and tremendously valuable. High-resolution crystal structures of GFP offer unprecedented opportunities to understand and manipulate the rela- tion between protein structure and spectroscopic function. GFP has become well established as a marker of gene expression and protein targeting in intact cells and organisms. Mutagenesis and engineering of GFP into chimeric proteins are opening new vistas in physiological indicators, biosensors, and photochemical memories. CONTENTS NATURAL AND SCIENTIFIC HISTORY OF GFP .................................510 Discovery and Major Milestones .............................................510 Occurrence, Relation to Bioluminescence, and Comparison with Other Fluorescent Proteins .....................................511 PRIMARY, SECONDARY, TERTIARY, AND QUATERNARY STRUCTURE ...........512 Primary Sequence from -

Protocol 4253-096-K
IFU0132 Rev 1 Status: RELEASED printed 12/8/2016 2:11:21 PM by Trevigen Document Control Instructions For Research Use Only. Not For Use In Diagnostic Procedures ® CometAssay 96 Reagent Kit for Higher Throughput Single Cell Gel Electrophoresis Assay (96-well) Catalog # 4253-096-K IFU0132 Rev 1 Status: RELEASED printed 12/8/2016 2:11:21 PM by Trevigen Document Control ® CometAssay 96 Reagent Kit for Higher Throughput Single Cell Gel Electrophoresis Assay (96-well) Catalog # 4253-096-K Table of Contents Page Number I. Background 1 II. Precautions and Limitations 1 III. Materials Supplied 2 IV. Materials Required But Not Supplied 2 V. Reagent Preparation 2 VI. Sample Preparation and Storage 4 VII. Assay Protocol 6 VIII. Data Analysis 7 IX. References 9 X. Related Products Available From Trevigen 10 XI. Appendices 12 XII. Troubleshooting Guide 13 © 2012 Trevigen, Inc. All rights reserved. Trevigen and CometAssay are registered trademarks, and CometSlide and FLARE are trademarks of Trevigen, Inc. i IFU0132 Rev 1 Status: RELEASED printed 12/8/2016 2:11:21 PM by Trevigen Document Control I. Background Trevigen’s CometAssay®, or single cell gel electrophoresis assay, provides a simple and effective method for evaluating DNA damage in cells. The principle of the assay is based upon the ability of denatured, cleaved DNA fragments to migrate out of the nucleoid under the influence of an electric field, whereas undamaged DNA migrates slower and remains within the confines of the nucleoid when a current is applied. Evaluation of the DNA “comet” tail shape and migration pattern allows for assessment of DNA damage. -

Microenvironment-Triggered Dual-Activation of a Photosensitizer
www.nature.com/scientificreports OPEN Microenvironment‑triggered dual‑activation of a photosensitizer‑ fuorophore conjugate for tumor specifc imaging and photodynamic therapy Chang Wang1, Shengdan Wang1, Yuan Wang1, Honghai Wu1, Kun Bao2, Rong Sheng1* & Xin Li1* Photodynamic therapy is attracting increasing attention, but how to increase its tumor‑specifcity remains a daunting challenge. Herein we report a theranostic probe (azo‑pDT) that integrates pyropheophorbide α as a photosensitizer and a NIR fuorophore for tumor imaging. The two functionalities are linked with a hypoxic‑sensitive azo group. Under normal conditions, both the phototoxicity of the photosensitizer and the fuorescence of the fuorophore are inhibited. While under hypoxic condition, the reductive cleavage of the azo group will restore both functions, leading to tumor specifc fuorescence imaging and phototoxicity. The results showed that azo‑PDT selectively images BEL‑7402 cells under hypoxia, and simultaneously inhibits BEL‑7402 cell proliferation after near‑infrared irradiation under hypoxia, while little efect on BEL‑7402 cell viability was observed under normoxia. These results confrm the feasibility of our design strategy to improve the tumor‑ targeting ability of photodynamic therapy, and presents azo‑pDT probe as a promising dual functional agent. Cancer is one of the most common causes of death, and more and more therapeutic strategies against this fatal disease have emerged in the past few decades. Among these strategies, photodynamic therapy has attracted much attention1. Tis therapy is based on singlet oxygen produced by photosensitizers under the irradiation with light of a specifc wavelength to damage tumor tissues (Fig. 1a). Since the photo-damaging efect is induced by the interaction between a photosensitizer and light, tumor-specifc therapy may be realized by focusing the light to the tumor site. -

Fluorescent Protein-Based Tools for Neuroscience
!1 !2 Fluorescent protein-based tools Outline for neuroscience An animatd primer on biosensor development Fluorescent proteins (FPs) Robert E. Campbell Department of Chemistry Other fluorophore technologies Single FP-based biosensors Imaging Structure & Function in the Nervous System Cold Spring Harbor, July 31, 2019. Lots of structural Lots of structural information information & Transmitted light Fluorescence microscopy of fluorescent color microscopy of live cells live cells No molecular provides molecular information information (more colors = more information) !5 !6 Fluorescence microscopy requires fluorophores Non-natural fluorophores for protein labelling Trends Bioch. Sci., 1984, 9, 88-91. O O O N O N 495 nm 519 nm 557 nm 576 nm - - CO2 CO2 S O O O N O N C N N C S ϕ = quantum yield - - ε = extinction coefficient CO2 CO2 ϕ Brightness ~ * ε S C i.e., for fluorescein N Proteins of interest ϕ = 0.92 N Fluorescein Tetramethylrhodamine ε = 73,000 M-1cm-1 C S (FITC) (TRITC) A non-natural fluorophore must be chemically linked to Non-natural fluorophores made by chemical synthesis a protein of interest… !7 !8 Getting non-natural fluorophores into a cell Some sea creatures make natural fluorophores Trends Bioch. Sci., 1984, 9, 88-91. O O O N O N Bioluminescent - Fluorescent CO2 CO -- S 2 S C N NHN C H S S Chemically labeled proteins of interest Microinjection with micropipet O O O N O N Fluorescent - CO2 CO - S 2 N NH H S …and then manually injected into a cell Some natural fluorophores are genetically encoded proteins http://www.luminescentlabs.org/and can be transplanted into cells as DNA! 228 OSAMU SHIMOMURA, FRANK H. -

Comparison of SYBR Green I and SYBR Gold Stains for Enumerating Bacteria and Viruses by Epifluorescence Microscopy
AQUATIC MICROBIAL ECOLOGY Vol. 43: 223–231, 2006 Published July 19 Aquat Microb Ecol Comparison of SYBR Green I and SYBR Gold stains for enumerating bacteria and viruses by epifluorescence microscopy Akira Shibata1, 4,*, Yoichi Goto1, Hiroaki Saito2, Tomohiko Kikuchi3, Tatuki Toda1, Satoru Taguchi1 1Faculty of Engineering, Soka University, 1-236 Tangi-cho, Hachioji, Tokyo 192-8577, Japan 2 Tohoku National Fisheries Research Institute, Shinhama-cho 3-27-5, Shiogama 985-0001, Japan 3Faculty of Education and Human Sciences, Yokohama National University, 792 Tokiwadai, Hodogaya, Yokohama 240-8501, Japan 4Present address: Department of Aquatic Bioscience, Graduate School of Agricultural and Life Sciences, University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan ABSTRACT: SYBR Gold staining is used for enumerating bacteria and viruses in aquatic samples. However, its suitability for epifluorescence microscopy has not been sufficiently investigated. Thus we compared bacterial and viral counts using SYBR Gold and SYBR Green I stains. Variables for both bacterial and viral counts included season and ocean depths of sample collection and the period of sustained excitation under epifluorescence microscopy. We also examined the storage period and procedures for preservation of samples with formaldehyde for bacterial counts. Natural seawater samples were used for all experiments. Ratios of counts obtained with SYBR Gold to those with SYBR Green I staining were 0.99 ± 0.09 (mean ± SD, n = 58) for bacteria and 1.0 ± 0.1 (n = 38) for viruses, which indicated no significant differences between stains. In samples fixed with 0.74% formalde- hyde that were stored at 4°C, bacterial counts obtained with SYBR Gold staining decreased over time in parallel with those obtained with SYBR Green I staining. -

Water-Soluble Pyrrolopyrrole Cyanine (Ppcy) NIR Fluorophores† Cite This: Chem
Erschienen in: Chemical Communications ; 2014, 50. - S. 4755-4758 ChemComm View Article Online COMMUNICATION View Journal | View Issue Water-soluble pyrrolopyrrole cyanine (PPCy) NIR fluorophores† Cite this: Chem. Commun., 2014, 50, 4755 Simon Wiktorowski, Christelle Rosazza, Martin J. Winterhalder, Ewald Daltrozzo Received 7th February 2014, and Andreas Zumbusch* Accepted 21st March 2014 DOI: 10.1039/c4cc01014k www.rsc.org/chemcomm Water-soluble derivatives of pyrrolopyrrole cyanines (PPCys) have been dyes, BODIPYs or others.7 Notable are also advances in other fields, synthesized by a post-synthetic modification route. In highly polar like the engineering of GFP-related fluorescing proteins or quantum media, these dyes are excellent NIR fluorophores. Labeling experiments dots, which have resulted in the synthesis of novel systems with NIR show how these novel dyes are internalized into mammalian cells. emission.8 To date, however, only a few water-soluble dyes with strong NIR absorptions and emissions have been known. Apart from the Near-infrared (NIR) light absorbing and emitting compounds have general scarcity of NIR absorbing molecules, the main reason for this attracted a lot of interest since the 1990’s.1 Initially, this was motivated is that NIR absorption is commonly observed in extended p-systems by their use in optical data storage or as laser dyes. Recently, however, which most often are hydrophobic. The incorporation of hydrophilic new applications of NIR dyes have emerged, which has led to a surge of functionalities into -

Chemical Probes to Visualize Bacterial Cell Structure and Physiology
molecules Review From Differential Stains to Next Generation Physiology: Chemical Probes to Visualize Bacterial Cell Structure and Physiology Jonathan Hira 1, Md. Jalal Uddin 1 , Marius M. Haugland 2 and Christian S. Lentz 1,* 1 Research Group for Host-Microbe Interactions, Department of Medical Biology and Centre for New Antibacterial Strategies (CANS), UiT—The Arctic University of Norway, 9019 Tromsø, Norway; [email protected] (J.H.); [email protected] (M.J.U.) 2 Department of Chemistry and Centre for New Antibacterial Strategies (CANS), UiT—The Arctic University of Norway, 9019 Tromsø, Norway; [email protected] * Correspondence: [email protected] Academic Editor: Steven Verhelst Received: 30 September 2020; Accepted: 23 October 2020; Published: 26 October 2020 Abstract: Chemical probes have been instrumental in microbiology since its birth as a discipline in the 19th century when chemical dyes were used to visualize structural features of bacterial cells for the first time. In this review article we will illustrate the evolving design of chemical probes in modern chemical biology and their diverse applications in bacterial imaging and phenotypic analysis. We will introduce and discuss a variety of different probe types including fluorogenic substrates and activity-based probes that visualize metabolic and specific enzyme activities, metabolic labeling strategies to visualize structural features of bacterial cells, antibiotic-based probes as well as fluorescent conjugates to probe biomolecular uptake pathways. Keywords: activity-based probe; antibiotic conjugate; bacterial imaging; bacterial uptake; fluorogenic substrate; metabolic labeling; phenotypic heterogeneity 1. Introduction—From 19th Century Microbiology to Modern Day Chemical Biology If chemical biology can be defined as the ‘interrogation of biological systems with chemical approaches’ [1], we must acknowledge some of the first microbiologists as chemical biologists. -

Near-Infrared Genetically Encoded Positive Calcium Indicator Based on GAF-FP Bacterial Phytochrome
Near-infrared genetically encoded positive calcium indicator based on GAF-FP bacterial phytochrome The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Subach,Oksana M., Natalia V. Barykina, Konstantin V. Anokhin, Kiryl D. Piatkevich, and Fedor V. Subach, "Near-infrared genetically encoded positive calcium indicator based on GAF-FP bacterial phytochrome." International Journal of Molecular Sciences 20, 14 (July 2019): no. 3488 doi 10.3390/ijms20143488 ©2019 Author(s) As Published 10.3390/ijms20143488 Publisher Multidisciplinary Digital Publishing Institute Version Final published version Citable link https://hdl.handle.net/1721.1/125333 Terms of Use Creative Commons Attribution Detailed Terms https://creativecommons.org/licenses/by/4.0/ International Journal of Molecular Sciences Article Near-Infrared Genetically Encoded Positive Calcium Indicator Based on GAF-FP Bacterial Phytochrome 1, 2, 2,3 Oksana M. Subach y , Natalia V. Barykina y , Konstantin V. Anokhin , Kiryl D. Piatkevich 4 and Fedor V. Subach 1,* 1 National Research Center “Kurchatov Institute”, Moscow 123182, Russia 2 P.K. Anokhin Institute of Normal Physiology, Moscow 125315, Russia 3 Lomonosov Moscow State University, Moscow 119991, Russia 4 MIT Media Lab, Massachusetts Institute of Technology, Cambridge, MA 02139-4307, USA * Correspondence: [email protected]; Tel.: +07-968-962-7083 These authors contributed equally to this work. y Received: 31 May 2019; Accepted: 15 July 2019; Published: 16 July 2019 Abstract: A variety of genetically encoded calcium indicators are currently available for visualization of calcium dynamics in cultured cells and in vivo. Only one of them, called NIR-GECO1, exhibits fluorescence in the near-infrared region of the spectrum. -

Orientation of Cyanine Fluorophores Terminally Attached to DNA Via Long, Flexible Tethers
1148 Biophysical Journal Volume 101 September 2011 1148–1154 Orientation of Cyanine Fluorophores Terminally Attached to DNA via Long, Flexible Tethers Jonathan Ouellet, Stephanie Schorr, Asif Iqbal, Timothy J. Wilson, and David M. J. Lilley* Cancer Research UK Nucleic Acid Structure Research Group, The University of Dundee, Dundee, United Kingdom ABSTRACT Cyanine fluorophores are commonly used in single-molecule FRET experiments with nucleic acids. We have previously shown that indocarbocyanine fluorophores attached to the 50-termini of DNA and RNA via three-carbon atom linkers stack on the ends of the helix, orienting their transition moments. We now investigate the orientation of sulfoindocarbocyanine fluorophores tethered to the 50-termini of DNA via 13-atom linkers. Fluorescence lifetime measurements of sulfoindocarbocya- nine 3 attached to double-stranded DNA indicate that the fluorophore is extensively stacked onto the terminal basepair at 15C, with properties that depend on the terminal sequence. In single molecules of duplex DNA, FRET efficiency between sulfoindo- carbocyanine 3 and 5 attached in this manner is modulated with helix length, indicative of fluorophore orientation and consistent with stacked fluorophores that can undergo lateral motion. We conclude that terminal stacking is an intrinsic property of the cyanine fluorophores irrespective of the length of the tether and the presence or absence of sulfonyl groups. However, compared to short-tether indocarbocyanine, the mean rotational relationship between the two fluorophores is changed by ~60 for the long- tether sulfoindocarbocyanine fluorophores. This is consistent with the transition moments becoming approximately aligned with the long axis of the terminal basepair for the long-linker species. -

Molecular and Spectroscopic Characterization of Green and Red Cyanine Fluorophores from the Alexa Fluor and AF Series
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381152; this version posted November 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Gebhardt et al., Molecular and spectroscopic characterization of green and red cyanine fluorophores from the Alexa Fluor and AF series Molecular and spectroscopic characterization of green and red cyanine fluorophores from the Alexa Fluor and AF series Christian Gebhardt1, Martin Lehmann2, Maria M. Reif3, Martin Zacharias3 & Thorben Cordes1,* 1 Physical and Synthetic Biology, Faculty of Biology, Ludwig-Maximilians-Universität München, Großhadernerstr. 2-4, 82152 Planegg-Martinsried, Germany 2 Plant Molecular Biology and Plant Metabolism, Faculty of Biology, Ludwig-Maximilians- Universität München, Großhadernerstr. 2-4, 82152 Planegg-Martinsried, Germany 3 Theoretical Biophysics (T38), Physics Department, Technical University of Munich, München, Germany corresponding author email: [email protected] Abstract The use of fluorescence techniques has had an enormous impact on various research fields including imaging, biochemical assays, DNA-sequencing and medical technologies. This has been facilitated by the availability of numerous commercial dyes, but often information about the chemical structures of dyes (and their linkers) are a well-kept secret. This can lead to problems for applications where a knowledge of the dye structure is necessary to predict (unwanted) dye-target interactions, or to establish structural models of the dye-target complex. Using a combination of spectroscopy, mass spectrometry and molecular dynamics simulations, we here investigate the molecular structures and spectroscopic properties of dyes from the Alexa Fluor (Alexa Fluor 555 and 647) and AF series (AF555, AF647, AFD647). -

Fluorescence Spectroscopy of Exogenous, Exogenously-Induced and Endogenous Fluorophores for the Photodetection and Photodynamic Therapy of Cancer
FLUORESCENCE SPECTROSCOPY OF EXOGENOUS, EXOGENOUSLY-INDUCED AND ENDOGENOUS FLUOROPHORES FOR THE PHOTODETECTION AND PHOTODYNAMIC THERAPY OF CANCER. Thèse présentée au Département de Génie Rural Ecole Polytechnique Fédérale de Lausanne par Matthieu Zellweger Jury: Dr Georges Wagnières, rapporteur Prof. Hubert van den Bergh, corapporteur Dr Christian Depeursinge, corapporteur Prof. René Salathé, corapporteur Prof. Philippe Monnier, corapporteur Prof. Stanley Brown, corapporteur Lausanne, Février 2000 2 Foreword This work is the final report about my research activity as a Ph.D. student in the LPAS laboratory of Prof. Hubert van den Bergh, at the Swiss Federal Institute of Technology - Lausanne (EPFL). Some parts of this report have been published or submitted during the course of my Ph.D. thesis. I therefore included the text of the publications instead of an original chapter whenever relevant. In such a case, the reference is clearly stated. A brief introduction always precedes the resulting 'chapters'. Due to this inclusion, the reader might find some redundant information within this report. On the other hand, it should be emphasized that each chapter can be read as a stand-alone text and need not be linked to any other part of the whole work. However, I tried to avoid the unnecessary repetition of information and this is especially true for the references. Whenever the reader feels that a reference should have been included to support an information in the introduction, they should check the introduction of the following published chapter for a more detailed reference list. The same criticism applies to the introduction and, again, the reader should check the published section of a chapter if they feel that it lacks some information.