Direct Visualization and Mapping of the Spatial Course of Fiber Tracts at Microscopic Resolution in the Human Hippocampus Downloaded from Michael M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Targeted Dorsal Dentate Gyrus Or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis
biology Article Targeted Dorsal Dentate Gyrus or Whole Brain Irradiation in Juvenile Mice Differently Affects Spatial Memory and Adult Hippocampal Neurogenesis Céline Serrano 1, Morgane Dos Santos 2, Dimitri Kereselidze 1, Louison Beugnies 1, Philippe Lestaevel 1, Roseline Poirier 3,*,† and Christelle Durand 1,*,† 1 Laboratory of Experimental Radiotoxicology and Radiobiology (LRTOX), Research Department on the Biological and Health Effects of Ionizing Radiation (SESANE), Institute for Radiological Protection and Nuclear Safety (IRSN), 92260 Fontenay-aux-Roses, France; [email protected] (C.S.); [email protected] (D.K.); [email protected] (L.B.); [email protected] (P.L.) 2 Laboratory of Radiobiology of Accidental Exposure (LRAcc), Research Department in Radiobiology and Regenerative Medicine (SERAMED), Institute for Radiological Protection and Nuclear Safety (IRSN), 92260 Fontenay-aux-Roses, France; [email protected] 3 Paris-Saclay Neuroscience Institute (Neuro-PSI), University Paris-Saclay, UMR 9197 CNRS, F-91405 Orsay, France * Correspondence: [email protected] (R.P.); [email protected] (C.D.) † These authors contribute equally to this work. Simple Summary: The effects of exposure of the juvenile brain to doses of ionizing radiation Citation: Serrano, C.; Dos Santos, M.; (IR) ≤ 2 Gy on cognitive functions in adulthood are not clearly established in humans, and exper- Kereselidze, D.; Beugnies, L.; imental data are scarce. To elucidate how IR can impact the postnatal brain, we evaluated and Lestaevel, P.; Poirier, R.; Durand, C. compared the effect of whole brain (WB) or hippocampal dorsal dentate gyrus (DDG) X-ray exposure Targeted Dorsal Dentate Gyrus or (0.25–2 Gy) on spatial memory, three months after irradiation in mice. -

Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment
The Journal of Neuroscience, December 1989, 9(12): 4355-4370 Lesions of Perirhinal and Parahippocampal Cortex That Spare the Amygdala and Hippocampal Formation Produce Severe Memory Impairment Stuart Zola-Morgan,’ Larry Ft. Squire,’ David G. Amaral,2 and Wendy A. Suzuki2J Veterans Administration Medical Center, San Diego, California, 92161, and Department of Psychiatry, University of California, San Diego, La Jolla, California 92093, The Salk Institute, San Diego, California 92136, and 3Group in Neurosciences, University of California, San Diego, La Jolla, California 92093 In monkeys, bilateral damage to the medial temporal region Moss, 1984). (In this notation, H refers to the hippocampus, A produces severe memory impairment. This lesion, which in- to the amygdala, and the plus superscript (+) to the cortical cludes the hippocampal formation, amygdala, and adjacent tissue adjacent to each structure.) This lesion appears to con- cortex, including the parahippocampal gyrus (the H+A+ le- stitute an animal model of medial temporal lobe amnesia like sion), appears to constitute an animal model of human me- that exhibited by the well-studied patient H.M. (Scoville and dial temporal lobe amnesia. Reexamination of histological Milner, 1957). material from previously studied monkeys with H+A+ lesions The H+A+ lesion produces greater memory impairment than indicated that the perirhinal cortex had also sustained sig- a lesion limited to the hippocampal formation and parahip- nificant damage. Furthermore, recent neuroanatomical stud- pocampal cortex-the H+ lesion (Mishkin, 1978; Mahut et al., ies show that the perirhinal cortex and the closely associated 1982; Zola-Morgan and Squire, 1985, 1986; Zola-Morgan et al., parahippocampal cortex provide the major source of cortical 1989a). -

Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: a Steered-Glutamatergic Perspective
brain sciences Review Neuromodulators and Long-Term Synaptic Plasticity in Learning and Memory: A Steered-Glutamatergic Perspective Amjad H. Bazzari * and H. Rheinallt Parri School of Life and Health Sciences, Aston University, Birmingham B4 7ET, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-(0)1212044186 Received: 7 October 2019; Accepted: 29 October 2019; Published: 31 October 2019 Abstract: The molecular pathways underlying the induction and maintenance of long-term synaptic plasticity have been extensively investigated revealing various mechanisms by which neurons control their synaptic strength. The dynamic nature of neuronal connections combined with plasticity-mediated long-lasting structural and functional alterations provide valuable insights into neuronal encoding processes as molecular substrates of not only learning and memory but potentially other sensory, motor and behavioural functions that reflect previous experience. However, one key element receiving little attention in the study of synaptic plasticity is the role of neuromodulators, which are known to orchestrate neuronal activity on brain-wide, network and synaptic scales. We aim to review current evidence on the mechanisms by which certain modulators, namely dopamine, acetylcholine, noradrenaline and serotonin, control synaptic plasticity induction through corresponding metabotropic receptors in a pathway-specific manner. Lastly, we propose that neuromodulators control plasticity outcomes through steering glutamatergic transmission, thereby gating its induction and maintenance. Keywords: neuromodulators; synaptic plasticity; learning; memory; LTP; LTD; GPCR; astrocytes 1. Introduction A huge emphasis has been put into discovering the molecular pathways that govern synaptic plasticity induction since it was first discovered [1], which markedly improved our understanding of the functional aspects of plasticity while introducing a surprisingly tremendous complexity due to numerous mechanisms involved despite sharing common “glutamatergic” mediators [2]. -

Of Severe Jaundice of the Newborn by L
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.18.1.17 on 1 February 1955. Downloaded from J. Neurol. Neurosurg. Psychiat., 1955, 18, 17. MORPHOLOGICAL NERVOUS CHANGES IN SURVIVORS OF SEVERE JAUNDICE OF THE NEWBORN BY L. CROME From the Neuropathology Department, the Fountain Hospital, London The clinical sequelae of Rh sensitization have the neonatal period with hydrops foetalis or, more been fully described by Pentschew (1948), Pickles commonly, icterus gravis, and, on the other hand, (1949), and Evans and Polani (1950). The clinical on a small total of 17 morphological descriptions variability of the condition is well known, but it of cases of established residual kemicterus, some tends to follow, nevertheless, a certain pattern. A of which were of doubtful aetiology. stormy neonatal period with jaundice, drowsiness, The structural changes found in the early fatal feeding difficulties, opisthotonos, and respiratory cases of Rh sensitization are characterized by disturbances is usually followed between the ages of generalized jaundice with staining by bilirubin of 1 and 2 years by a "silent" period. After that, certain focal areas of the grey matter of the central guest. Protected by copyright. rigidity, intermittent opisthotonos, and involuntary nervous system, a blood picture marked by erythro- movements make their appearance. Hypertonia blastosis and haemolysis, and some less constant may be present and this may be replaced by the findings in other organs such as persistence of intermittent spasms of athetosis. Involuntary haemopoietic foci in the liver and spleen, increase choreiform movements may set in later, and deafness in size and weight of the liver and spleen, and the is also common. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Glutamate Binding in Primate Brain
Journal of Neuroscience Research 27512-521 (1990) Quisqualate- and NMDA-Sensitive [3H]Glutamate Binding in Primate Brain A.B. Young, G.W. Dauth, Z. Hollingsworth, J.B. Penney, K. Kaatz, and S. Gilman Department of Neurology. University of Michigan, Ann Arbor Excitatory amino acids (EAA) such as glutamate and Young and Fagg, 1990). Because EAA are involved in aspartate are probably the neurotransmitters of a general cellular metabolic functions, they were not orig- majority of mammalian neurons. Only a few previous inally considered to be likcl y neurotransmitter candi- studies have been concerned with the distribution of dates. Electrophysiological studies demonstrated con- the subtypes of EAA receptor binding in the primate vincingly the potency of these substances as depolarizing brain. We examined NMDA- and quisqualate-sensi- agents and eventually discerned specific subtypes of tive [3H]glutamate binding using quantitative autora- EAA receptors that responded preferentially to EAA an- diography in monkey brain (Macaca fascicularis). alogs (Dingledine et al., 1988). Coincident with the elec- The two types of binding were differentially distrib- trophysiological studies, biochemical studies indicated uted. NMDA-sensitive binding was most dense in that EAA were released from slice and synaptosome dentate gyrus of hippocampus, stratum pyramidale preparations in a calcium-dependent fashion and were of hippocampus, and outer layers of cerebral cortex. accumulated in synaptosomc preparations by a high-af- Quisqualate-sensitive binding was most dense in den- finity transport system. With these methodologies, EAA tate gyrus of hippocampus, inner and outer layers of release and uptake were found to be selectively de- cerebral cortex, and molecular layer of cerebellum. -
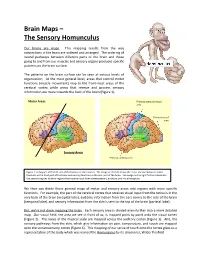
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Neural Populations in Human Posteromedial Cortex Display Opposing Responses During Memory and Numerical Processing
Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing Brett L. Foster, Mohammad Dastjerdi, and Josef Parvizi1 Laboratory of Behavioral and Cognitive Neurology, Department of Neurology and Neurological Sciences, Stanford Human Intracranial Cognitive Electrophysiology Program, Stanford University School of Medicine, Stanford University, Stanford, CA 94305 Edited by Marcus E. Raichle, Washington University in St. Louis, St. Louis, MO, and approved August 7, 2012 (received for review April 23, 2012) Our understanding of the human default mode network derives rate is correlated with behavioral performance, where lower levels primarily from neuroimaging data but its electrophysiological of PCC firing suppression are associated with longer reaction correlates remain largely unexplored. To address this limitation, times (RTs) and higher error rate, a correlation also previously we recorded intracranially from the human posteromedial cortex reported with fMRI in humans (11, 12). Similarly, direct cortical (PMC), a core structure of the default mode network, during various recordings using electrocorticography (ECoG) from human conditions of internally directed (e.g., autobiographical memory) as DMN (13–16) have shown suppression of broadband γ-power [a opposed to externally directed focus (e.g., arithmetic calculation). correlate of population firing rate (17, 18)] during attentional We observed late-onset (>400 ms) increases in broad high γ-power effort. More recently, this broadband suppression across human (70–180 Hz) within PMC subregions during memory retrieval. High DMN regions has also been shown to correlate with behavioral γ-power was significantly reduced or absent when subjects re- performance (16). Taken together, these findings have provided trieved self-referential semantic memories or responded to self- clear electrophysiological evidence for DMN suppression. -

Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: a Comparative in Situ Hybridization Analysis
The Journal of Neuroscience, March 1993. 73(3): 1258-1279 Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: A Comparative in situ Hybridization Analysis Martin K.-H. Schafer,i-a Robert Day,* William E. Cullinan,’ Michel Chri?tien,3 Nabil G. Seidah,* and Stanley J. Watson’ ‘Mental Health Research Institute, University of Michigan, Ann Arbor, Michigan 48109-0720 and J. A. DeSeve Laboratory of *Biochemical and 3Molecular Neuroendocrinology, Clinical Research Institute of Montreal, Montreal, Quebec, Canada H2W lR7 Posttranslational processing of proproteins and prohor- The participation of neuropeptides in the modulation of a va- mones is an essential step in the formation of bioactive riety of CNS functions is well established. Many neuropeptides peptides, which is of particular importance in the nervous are synthesized as inactive precursor proteins, which undergo system. Following a long search for the enzymes responsible an enzymatic cascade of posttranslational processing and mod- for protein precursor cleavage, a family of Kexin/subtilisin- ification events during their intracellular transport before the like convertases known as PCl, PC2, and furin have recently final bioactive products are secreted and act at either pre- or been characterized in mammalian species. Their presence postsynaptic receptors. Initial endoproteolytic cleavage occurs in endocrine and neuroendocrine tissues has been dem- C-terminal to pairs of basic amino acids such as lysine-arginine onstrated. This study examines the mRNA distribution of (Docherty and Steiner, 1982) and is followed by the removal these convertases in the rat CNS and compares their ex- of the basic residues by exopeptidases. Further modifications pression with the previously characterized processing en- can occur in the form of N-terminal acetylation or C-terminal zymes carboxypeptidase E (CPE) and peptidylglycine a-am- amidation, which is essential for the bioactivity of many neu- idating monooxygenase (PAM) using in situ hybridization ropeptides.