Reproductive Potential and Minimum Reproductive Size of Ferocactus Wislizeni (Cactaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pima County Plant List (2020) Common Name Exotic? Source
Pima County Plant List (2020) Common Name Exotic? Source McLaughlin, S. (1992); Van Abies concolor var. concolor White fir Devender, T. R. (2005) McLaughlin, S. (1992); Van Abies lasiocarpa var. arizonica Corkbark fir Devender, T. R. (2005) Abronia villosa Hariy sand verbena McLaughlin, S. (1992) McLaughlin, S. (1992); Van Abutilon abutiloides Shrubby Indian mallow Devender, T. R. (2005) Abutilon berlandieri Berlandier Indian mallow McLaughlin, S. (1992) Abutilon incanum Indian mallow McLaughlin, S. (1992) McLaughlin, S. (1992); Van Abutilon malacum Yellow Indian mallow Devender, T. R. (2005) Abutilon mollicomum Sonoran Indian mallow McLaughlin, S. (1992) Abutilon palmeri Palmer Indian mallow McLaughlin, S. (1992) Abutilon parishii Pima Indian mallow McLaughlin, S. (1992) McLaughlin, S. (1992); UA Abutilon parvulum Dwarf Indian mallow Herbarium; ASU Vascular Plant Herbarium Abutilon pringlei McLaughlin, S. (1992) McLaughlin, S. (1992); UA Abutilon reventum Yellow flower Indian mallow Herbarium; ASU Vascular Plant Herbarium McLaughlin, S. (1992); Van Acacia angustissima Whiteball acacia Devender, T. R. (2005); DBGH McLaughlin, S. (1992); Van Acacia constricta Whitethorn acacia Devender, T. R. (2005) McLaughlin, S. (1992); Van Acacia greggii Catclaw acacia Devender, T. R. (2005) Acacia millefolia Santa Rita acacia McLaughlin, S. (1992) McLaughlin, S. (1992); Van Acacia neovernicosa Chihuahuan whitethorn acacia Devender, T. R. (2005) McLaughlin, S. (1992); UA Acalypha lindheimeri Shrubby copperleaf Herbarium Acalypha neomexicana New Mexico copperleaf McLaughlin, S. (1992); DBGH Acalypha ostryaefolia McLaughlin, S. (1992) Acalypha pringlei McLaughlin, S. (1992) Acamptopappus McLaughlin, S. (1992); UA Rayless goldenhead sphaerocephalus Herbarium Acer glabrum Douglas maple McLaughlin, S. (1992); DBGH Acer grandidentatum Sugar maple McLaughlin, S. (1992); DBGH Acer negundo Ashleaf maple McLaughlin, S. -

Responses of Plant Communities to Grazing in the Southwestern United States Department of Agriculture United States Forest Service
Responses of Plant Communities to Grazing in the Southwestern United States Department of Agriculture United States Forest Service Rocky Mountain Research Station Daniel G. Milchunas General Technical Report RMRS-GTR-169 April 2006 Milchunas, Daniel G. 2006. Responses of plant communities to grazing in the southwestern United States. Gen. Tech. Rep. RMRS-GTR-169. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p. Abstract Grazing by wild and domestic mammals can have small to large effects on plant communities, depend- ing on characteristics of the particular community and of the type and intensity of grazing. The broad objective of this report was to extensively review literature on the effects of grazing on 25 plant commu- nities of the southwestern U.S. in terms of plant species composition, aboveground primary productiv- ity, and root and soil attributes. Livestock grazing management and grazing systems are assessed, as are effects of small and large native mammals and feral species, when data are available. Emphasis is placed on the evolutionary history of grazing and productivity of the particular communities as deter- minants of response. After reviewing available studies for each community type, we compare changes in species composition with grazing among community types. Comparisons are also made between southwestern communities with a relatively short history of grazing and communities of the adjacent Great Plains with a long evolutionary history of grazing. Evidence for grazing as a factor in shifts from grasslands to shrublands is considered. An appendix outlines a new community classification system, which is followed in describing grazing impacts in prior sections. -

Begin Typing Here
December 16, 2013 Kimberly D. Bose, Secretary Federal Energy Regulatory Commission 888 First Street NE, Room 1A Washington, DC 20426 Re: Sierrita Pipeline Project Draft Environmental Impact Statement Dear Secretary Bose: The Arizona Game and Fish Department (Department), along with the U.S. Fish and Wildlife Service, Buenos Aires National Wildlife Refuge (BANWR), and U.S. Customs and Border Protection, is a cooperating agency with the Federal Energy Regulatory Commission (FERC) for the National Environmental Policy Act (NEPA) analysis of the project. We have participated in the planning and review of this project with the FERC, Sierrita Gas Pipeline LLC (Sierrita), and the other cooperating agencies since early 2012. Although Sierrita has submitted to the FERC a considerable amount of detailed information pertaining to the design, construction, and post- construction restoration of the pipeline, there remains a lack of sufficient detail in some of Sierrita’s plans to engender confidence that the pipeline will not result in unmitigated impacts to wildlife habitat in the Altar Valley. Upon review of the Draft Environmental Impact Statement (EIS), the Department submits for your consideration a number of recommendations we feel would help offset wildlife and habitat impacts that would result if the pipeline is certificated. To aid in your response to comments, this letter is structured such that supporting information for each major comment is presented in descriptive paragraphs with specific actions summarized in bolded, italicized bullets. MITIGATION There is no mitigation proposed for the 376.7 acres of permanent disturbance (i.e., acres of vegetation within the 50-foot-wide permanent ROW or occupied by aboveground facilities). -

Fishhook Barrel Or Biznaga De Agua Ferocactus Wislizeni
ARIZONA-SONORA DESERT MUSEUM PLANT CARE INFORMATION Fishhook Barrel or Biznaga de Agua Ferocactus wislizeni DESCRIPTION: Fishhook Barrel is found throughout the Arizona Upland portion of the Sonoran Desert, and higher into the grasslands and Chihuahuan Desert. It is has a slow to moderate growth rate, up to 5' or more tall, and 2' wide. It has a deeply pleated surface with stout, hooked spines which start red and fade to gray. Blooming begins in July or August and continues for up to 8 weeks. Flowers vary within the species from yellow, orange, red, to coppery. Many insect species visit the flowers, but bees are the main pollinators. Fruit ripen in late fall and winter. The rind is edible and has a lemony taste. The seeds are high in oil. An unusual form, CSSA Yellow Fishhook Barrel, is now available. Not only does it have bright yellow flowers, but the spines are yellow also. RECOMMENDED USE: Accent, container, desert enhancement or revegetation CULTURE: Hardiness: Fishhook barrel is hardy to at least 15oF. Sun tolerance: Full sun or light shade produce the healthiest plants and best flowering. Please note if there is an “S” marked on the pot or box. This denotes which side has been facing south in the nursery. Keep this orientation to prevent sunburning. Whether or not there is an “S”, we recommend that you acclimatize your plant gradually to the sun. Watering and feeding: For potted plants, regular watering during the warm season is required. It can be adapted to unwatered desert conditions in the ground, but will grow faster if given occasional soaks. -

Red Gap Ranch Biological Resource Evaluation
RED GAP RANCH BIOLOGICAL RESOURCE EVALUATION Prepared for: Southwest Ground-water Consultants, Inc. Prepared by: WestLand Resources, Inc. Date: February 14, 2014 Project No.: 1822.01 TABLE OF CONTENTS 1. BACKGROUND AND OBJECTIVES ................................................................................................ 1 2. EXISTING ENVIRONMENT AND BIOLOGICAL RESOURCES ................................................... 2 2.1. Approach ...................................................................................................................................... 2 2.2. Physical Environment ................................................................................................................... 2 2.3. Biological Environment and Resources ....................................................................................... 3 3. SCREENING ANALYSIS FOR SPECIES OF CONCERN ................................................................ 5 3.1. Approach ...................................................................................................................................... 5 3.2. Screening Analysis Results .......................................................................................................... 7 3.2.1. USFWS-listed Species ...................................................................................................... 7 3.2.2. USFS Coconino National Forest Sensitive Species ........................................................ 15 3.2.3. USFS Management Indicator Species ............................................................................ -

Ecological Site R041XB207AZ Limy Slopes 8-12" P.Z
Natural Resources Conservation Service Ecological site R041XB207AZ Limy Slopes 8-12" p.z. Last updated: 8/06/2020 Accessed: 09/27/2021 General information Provisional. A provisional ecological site description has undergone quality control and quality assurance review. It contains a working state and transition model and enough information to identify the ecological site. Figure 1. Mapped extent Areas shown in blue indicate the maximum mapped extent of this ecological site. Other ecological sites likely occur within the highlighted areas. It is also possible for this ecological site to occur outside of highlighted areas if detailed soil survey has not been completed or recently updated. MLRA notes Major Land Resource Area (MLRA): 041X–Southeastern Arizona Basin and Range AZ 41.2 – Chihuahuan – Sonoran Desert Shrubs Elevations range from 2600 to 4000 feet and precipitation ranges from 8 to 12 inches per year. Vegetation includes mesquite, palo verde, catclaw acacia, soaptree yucca, creosotebush, whitethorn, staghorn cholla, desert saltbush, Mormon tea, burroweed, snakeweed, tobosa, black grama, threeawns, bush muhly, dropseed, and burrograss. The soil temperature regime is thermic and the soil moisture regime is typic aridic. This unit occurs within the Basin and Range Physiographic Province and is characterized by numerous mountain ranges that rise abruptly from broad, plain-like valleys and basins. Igneous and metamorphic rock classes dominate the mountain ranges and sediments filling the basins represent combinations of fluvial, lacustrine, colluvial and alluvial deposits. Associated sites F041XB221AZ Loamy Bottom 8-12" p.z. woodland F041XB222AZ Saline Bottom 8-12" p.z. woodland R041XB206AZ Limy Fan 8-12" p.z. R041XB208AZ Limy Upland 8-12" p.z. -

What Is a Cactus?
Sandoval County Master Gardeners 3/16/2016 Cacti and Other Κακτος ------ prickly plant (thistle) Κακτος ------ prickly plant (thistle) Succulents of the Cacti are: Cacti are: Southwest Rich Reif 1. Succulents 1. Succulents March 16, 2016 2. New world plants --1-- --2-- --3-- --4-- --5-- --6-- 1 Sandoval County Master Gardeners 3/16/2016 --7-- --8-- --9-- --10-- --11-- --12-- --13-- --14-- --1-- 2 Sandoval County Master Gardeners 3/16/2016 --1-- Not a cactus! --2-- Euphorbia debilispina CACTUS!! --3-- CACTUS!! Pereskia aculeata Opuntia lepticaulis Barbados gooseberry; leaf cactus Pencil cholla; Desert Christmas cactus --4-- --5-- Not a cactus! Euphorbia mammillaris Corn cob cactus 3 Sandoval County Master Gardeners 3/16/2016 Not a cactus! --6-- CACTUS!! Agave toumeyana Ariocarpus agavoides Toumey’s agave Tamaulipas living rock --7-- CACTUS!! --8-- Ariocarpus fissuratus Living rock Not a cactus --9-- Not a cactus Euphorbia pseudocactus Pachypodium geayi Candelabra spurge; false cactus Madagascar palm 4 Sandoval County Master Gardeners 3/16/2016 --10-- CACTUS!! --11-- Tephrocactus articulatus Paper-spined cholla --12-- Not a cactus CACTUS!! Leuchtenbergia principis Agave cactus, Prism cactus Tromotriche revoluta (syn: Stapelia revoluta) Carrion plant (?) --13-- --14-- CACTUS!! Astrophytum myriostigma Bishop’s cap, Bishop’s hat 5 Sandoval County Master Gardeners 3/16/2016 Not a cactus Five characteristics of all cacti: Five characteristics of all cacti: 1. Perennial 1. Perennial 2. Dicot -- plant with two seed leaves Euphorbia trigona African milk tree, Cathedral cactus Spider plant Pereskia Iris Sycamore Five characteristics of all cacti: Pachypodium 1. Perennial Lily 2. Dicot -- plant with two seed leaves Apple Willow 3. Fruit is a berry Dicot leaves Monocot leaves Daylily Five characteristics of all cacti: Echinocereus dasyacanthus Rainbow cactus 1. -

Page 55 of Þÿ GAGEAGRANATELIICONTRI BU B IILACUNOA
Acta Horti Bot. Bucurest. 2018, 45: 47-55 EUPHORBIA GENUS IN BOTANICAL GARDEN IASSY – FEATURES OF LIVING PLANT COLLECTION IFRIM Camelia 1* Abstract: The Euphorbia genus collection from the Botanical Garden of Iassy comprises 28 taxa, of which 18 are originated in the dry areas of South Africa. Most of the South- African species have conservative value, belonging to different sozological categories, at national or international level. The experience of cultivating items from the collection led to the establishment of characteristics concerning cultivation particularities, which are useful for horticultural purposes. The morphological studies of the plants cultivated in GBI greenhouses put together with information from specialty literature have permitted the development of an artificial key, useful in determining species from the collection. Key words: Euphorbia genus, Botanical Garden Iassy, artificial taxonomic key Received 30 September 2018 Accepted 15 November 2018 Introduction The Euphorbia genus is considered the second richest in the angiosperms group, the number of circumscribed species are appreciated at approximately 2000 (Berry et al. 2016) or even 2250 (Esser 2009). The genus has a cosmopolitan distribution (except Antarctica), the majority of the species occurs in tropical and subtropical Africa and America. The habitats occupied by species are very diverse, from forest steppe to arid areas; biological forms are very diverse as well, from herbaceous annual plants to trees with impressive dimensions. Characteristic for this polyphyletic genus is the inflorescence structure, named cyathium; likewise spurges are recognized for their irritant latex (Carter & Leach 2001). The genus’ name seems to have come from the name Euphorbus (Euphorbos) of the Greek physician of Juba (Iuba) II, king of Mauritania (a Berber kingdom that was part of present-day western Algeria and northern Morocco) from first century AD (Smith 1849). -

A Phylogenetic Study of Ferocactus Britton and Rose (Cactaceae: Cactoideae) Jorge Hugo Cota-Sánchez Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1997 A phylogenetic study of Ferocactus Britton and Rose (Cactaceae: Cactoideae) Jorge Hugo Cota-Sánchez Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons, Other Ecology and Evolutionary Biology Commons, Other Genetics and Genomics Commons, and the Plant Breeding and Genetics Commons Recommended Citation Cota-Sánchez, Jorge Hugo, "A phylogenetic study of Ferocactus Britton and Rose (Cactaceae: Cactoideae) " (1997). Retrospective Theses and Dissertations. 11453. https://lib.dr.iastate.edu/rtd/11453 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfihn master. TJMI fihns the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. -

Desert Mule Deer, Pronghorn Antelope, Gambel's Quail, Scaled Quail, and Blacktailed Jackrabbit
Loamy Upland 12 – 16 PZ R041XC313AZ This site is important for many wildlife species. Major species include desert mule deer, pronghorn antelope, Gambel's quail, scaled quail, and blacktailed jackrabbit. This site has no natural surface water associated with it. Water developments are important to these and other wildlife on this site. Being an open grassland, this site is also home to a variety of small herbivores, birds, and their associated predators. With the exception of prong- horn antelope, this site is mainly a forage area for larger wildlife species. The value of this site for food or cover requirements for specific wildlife species changes with the changes in the vegetation that occur from one plant community to another. Each plant community and each animal species must be considered individually. Plant Preferences by Animal Kind Common name Scientific name Plant - - - - - - - - - - - - - - - - - - - Forage preferences* - - - - - - - - - - - - - - - - - - part J F M A M J J A S O N D Animal Kind: Cattle Sideoats grama Bouteloua curtipendula leaf DDDPPPPDDDDD Plains lovegrass Eragrostis intermedia entire DDDPPPPDDDDD Cane beardgrass Bothriochloa barbinoides leaf PPPPDDDDUUUU Blue grama Bouteloua gracilis leaf PPPPDDUUUUUU Sprucetop grama Bouteloua chondrosioides leaf PPPPPPPPPPPP Curly-mesquite Hilaria mutica leaf PPPNNUUUUUUU Hairy grama Bouteloua hirsuta leaf DDDDDDDUUUUU Spider grass Aristida ternipes leaf UUUUUUUUUDDD Red threeawn Aristida longiseta entire NNNNNNDDDDDN False mesquite Calliandra eriophylla leaf DDDDDDDDDDDD -
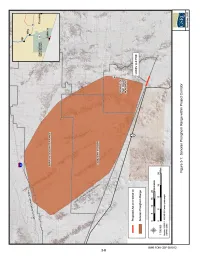
Pima County Is Included in Appendix B
The lesser long-nosed bat is found during the summer within desert grasslands and scrublands. The lesser long-nosed bat spends the day in caves and tunnels and forages at night upon plant nectar and pollen. This bat is an important pollinator of agave, and organ pipe and saguaro cacti (AGFD 2003). Roosting occurs in caves, abandoned buildings, and mines, which are usually located at the base of mountains where food sources are present (AGFD 2003). The lesser long-nosed bat is a seasonal resident of the OPCNM. Roosting sites are located in the OPCNM, but no known roosting sites occur within the project corridor (NPS 2003). The closest location of a known maternity colony to the project corridor would be approximately 15 miles (NPS 2003). 3.6.1.3 Acuña Cactus The candidate status of Acuña cactus was last reviewed on May 11, 2005 (70 FR 24870). Seven populations of Acuña cactus are currently known to exist (Baiza 2007). The species is restricted to well drained knolls and gravel ridges between major washes on substrates, including granite hills and flats and bright red to white andesite, occurring from 1,300 to 2,000 feet in elevation (AGFD 2004). The species requires insect vectors for pollination, with polylectic bee species being the primary agent (AGFD 2004). Dispersal occurs primarily through gravity, and secondarily by wind, rain, and small insects. As a candidate species, the Acuña cactus is not Federally protected, but is protected by the Arizona’s Native Plant Law. Consideration is given to candidate species because of the potential for their listing during project activities, which could require USFWS Section 7 consultation. -

Ferocactus Echinocactus 18Aug2013
The Weekly Plant 18 August 2013 Scientiic/Common names: Ferocactus wislizeni1/Arizona barrel cactus, ishhook barrel, compass barrel Echinocactus grusonii1/golden barrel cactus, golden ball, mother-in-law’s cushion TAV location: Arizona barrel - several plants are in the quadrangle between the gym/swimming pool and the Community Center. Golden barrel - small specimens in landscapes around the Village. Large, lowering specimens can be seen at B&B Cactus on Speedway. Ferocactus wislizeni, left, Discussion: Echinocactus grusonii, right. I’ve been hesitant to write about both barrels and saguaros. After all, what can I say that you haven’t read already? But, maybe you haven’t heard the botanical and taxonomic details. So, this week I’ll discuss two common barrel cactus. If you look at the scientiic name of different cactus2, you will see that some include the word “cactus” (like this week’s plants) or the word “cereus” (like the hedgehog cactus, Echinocereus fasciculatus, Weekly Plant 8 Apr2012). This is a clue about the plant’s lowering habit (if those words aren’t in the name, you have no clues about lowering). The “cactus” lower on the newer growth, near the tip of the plant (see photos above). The “cereus” lower on older growth - the lowers appear on the sides of the plant. Saguaro and hedgehog cactus are in this group. What more can we learn about this week’s plants - Ferocactus and Echinocactus? “Fero” is from the Latin ferus, meaning ierce or wild, for those wicked spines. “Echino” is from the Greek word for the spiny hedgehog, Echinocactus so named because of those wicked spines (that didn’t help much).