A Molecular, Morphological and Ecological Re-Appraisal of Venturiales―A New Order of Dothideomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

The Taxonomy, Phylogeny and Impact of Mycosphaerella Species on Eucalypts in South-Western Australia
The Taxonomy, Phylogeny and Impact of Mycosphaerella species on Eucalypts in South-Western Australia By Aaron Maxwell BSc (Hons) Murdoch University Thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy School of Biological Sciences and Biotechnology Murdoch University Perth, Western Australia April 2004 Declaration I declare that the work in this thesis is of my own research, except where reference is made, and has not previously been submitted for a degree at any institution Aaron Maxwell April 2004 II Acknowledgements This work forms part of a PhD project, which is funded by an Australian Postgraduate Award (Industry) grant. Integrated Tree Cropping Pty is the industry partner involved and their financial and in kind support is gratefully received. I am indebted to my supervisors Associate Professor Bernie Dell and Dr Giles Hardy for their advice and inspiration. Also, Professor Mike Wingfield for his generosity in funding and supporting my research visit to South Africa. Dr Hardy played a great role in getting me started on this road and I cannot thank him enough for opening my eyes to the wonders of mycology and plant pathology. Professor Dell’s great wit has been a welcome addition to his wealth of knowledge. A long list of people, have helped me along the way. I thank Sarah Jackson for reviewing chapters and papers, and for extensive help with lab work and the thinking through of vexing issues. Tania Jackson for lab, field, accommodation and writing expertise. Kar-Chun Tan helped greatly with the RAPD’s research. Chris Dunne and Sarah Collins for writing advice. -

Mycosphere Notes 225–274: Types and Other Specimens of Some Genera of Ascomycota
Mycosphere 9(4): 647–754 (2018) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/9/4/3 Copyright © Guizhou Academy of Agricultural Sciences Mycosphere Notes 225–274: types and other specimens of some genera of Ascomycota Doilom M1,2,3, Hyde KD2,3,6, Phookamsak R1,2,3, Dai DQ4,, Tang LZ4,14, Hongsanan S5, Chomnunti P6, Boonmee S6, Dayarathne MC6, Li WJ6, Thambugala KM6, Perera RH 6, Daranagama DA6,13, Norphanphoun C6, Konta S6, Dong W6,7, Ertz D8,9, Phillips AJL10, McKenzie EHC11, Vinit K6,7, Ariyawansa HA12, Jones EBG7, Mortimer PE2, Xu JC2,3, Promputtha I1 1 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand 2 Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming 650201, China 3 World Agro Forestry Centre, East and Central Asia, 132 Lanhei Road, Kunming 650201, Yunnan Province, People’s Republic of China 4 Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biological Resource and Food Engineering, Qujing Normal University, Qujing, Yunnan 655011, China 5 Shenzhen Key Laboratory of Microbial Genetic Engineering, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518060, China 6 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 7 Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand 8 Department Research (BT), Botanic Garden Meise, Nieuwelaan 38, BE-1860 Meise, Belgium 9 Direction Générale de l'Enseignement non obligatoire et de la Recherche scientifique, Fédération Wallonie-Bruxelles, Rue A. -

Molecular Systematics of the Marine Dothideomycetes
available online at www.studiesinmycology.org StudieS in Mycology 64: 155–173. 2009. doi:10.3114/sim.2009.64.09 Molecular systematics of the marine Dothideomycetes S. Suetrong1, 2, C.L. Schoch3, J.W. Spatafora4, J. Kohlmeyer5, B. Volkmann-Kohlmeyer5, J. Sakayaroj2, S. Phongpaichit1, K. Tanaka6, K. Hirayama6 and E.B.G. Jones2* 1Department of Microbiology, Faculty of Science, Prince of Songkla University, Hat Yai, Songkhla, 90112, Thailand; 2Bioresources Technology Unit, National Center for Genetic Engineering and Biotechnology (BIOTEC), 113 Thailand Science Park, Paholyothin Road, Khlong 1, Khlong Luang, Pathum Thani, 12120, Thailand; 3National Center for Biothechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, Maryland 20892-6510, U.S.A.; 4Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon, 97331, U.S.A.; 5Institute of Marine Sciences, University of North Carolina at Chapel Hill, Morehead City, North Carolina 28557, U.S.A.; 6Faculty of Agriculture & Life Sciences, Hirosaki University, Bunkyo-cho 3, Hirosaki, Aomori 036-8561, Japan *Correspondence: E.B. Gareth Jones, [email protected] Abstract: Phylogenetic analyses of four nuclear genes, namely the large and small subunits of the nuclear ribosomal RNA, transcription elongation factor 1-alpha and the second largest RNA polymerase II subunit, established that the ecological group of marine bitunicate ascomycetes has representatives in the orders Capnodiales, Hysteriales, Jahnulales, Mytilinidiales, Patellariales and Pleosporales. Most of the fungi sequenced were intertidal mangrove taxa and belong to members of 12 families in the Pleosporales: Aigialaceae, Didymellaceae, Leptosphaeriaceae, Lenthitheciaceae, Lophiostomataceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Testudinaceae and Trematosphaeriaceae. Two new families are described: Aigialaceae and Morosphaeriaceae, and three new genera proposed: Halomassarina, Morosphaeria and Rimora. -

Research Collection
Research Collection Doctoral Thesis Studies on Venturiaceae on Rosaceous plants Author(s): Menon, Radha Publication Date: 1956 Permanent Link: https://doi.org/10.3929/ethz-a-000092066 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library Diss ETH Prom. Nr. 2585 B Studies on Venturiaceae on Rosaceous Plants THESIS PRESENTED TO THE SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH FOR THE DEGREE OF DOCTOR OF NATURAL SCIENCES BY RADHA MENON at CITIZEN OF Ser\ INDIA Accepted on the recommendation of Prof. Dr. E. Gaumann and Prof. Dr. A. Frey-Wyssling 19 5 6 Druck von A. W. Hayn's Erben, Berlin SO 36 Veroffentlicht in „Phytopathologische Zcitschrift" Band 27, Heft 2, Seite 117 bis 146 (1956) Verlag Paul Parey, Berlin und Hamburg From the Department of special Botany of the Swiss Federal Institute of Technology in Zurich Director: Prof. Dr. E. Gdumann Studies on Venturiaceae on Rosaceous Plants By Radha Menon With 10 Figures Contents: I. General Introduction. A. Venturiaceae. B. Venturiaceae on Rosaceae: 1) Venturia, 2) Coleroa, 3) Gibbera, 4) Xenomeris, 5) Apiosporina. — II. Experimental Part. A. Cultural Studies. B. Inoculation Experiments: 1) Introduction, 2) Inoculation Studies, 3) Results, 4) Conclusions. — III. Morphological and Cultural Studies. A. Genus Venturia: 1) Venturia inaequalis, 2) Venturia tomentosae, 3) Venturia pirina, 4) Venturia pruni-cerasi, 5) Venturia Mullcri, 6) Venturia potentillae, 7) Venturia palustris, 8) Venturia alchemillae. — Appendix: Fusicladium eriobotryae. — B. Genus Coleroa: Coleroa chac- tomium. — C. Genus Gibbera: Gibbera rosae. -

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -
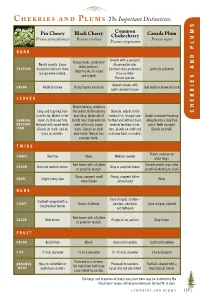
C P the Important Distinctions
C P The Important Distinctions S Common M Pin Cherry Black Cherry Chokecherry Canada Plum Prunus pennsylvanica Prunus serotina Prunus nigra U Prunus virginiana L P BARK D Smooth with a pungent, Young trunks: prominent N Nearly smooth. Large disagreeable odor. white lenticals. TEXTURE horizontal lenticels show Lenticels less prominent Lenticels yellowish A Older trunks: fissured orange when rubbed. than on other and ridged. Prunus species. S E Grayish-brown, with I COLOR Reddish-brown Young trunks are black Dull reddish-brown to black light-colored fissures R LEAVES R E Elliptic/oblong, widest in H Long and tapering from the center, thick leathery Obovate, widest in the C base to tip. Widest in the and shiny. Underside of terminal 1⁄3, sharply saw- Ovate or obovate tapering GENERAL lower 1⁄3; thin and firm midrib near stalk end cov- toothed and without hairs, abruptly into a long thin DESCRIP- textured with round teeth. ered with rusty, brown medium leathery in tex- point. Teeth rounded. TION Glands on stalk, and no hairs. Glands on stalk ture, glands on stalk and Glands on stalk. hairs on midribs. near blade. Margin has no brown hairs on midrib. rounded teeth. TWIGS Thorns common on SHAPE Very fine Waxy Medium slender older twigs Red-brown with a lighter Current growth gray, older COLOR Red and reddish-brown Gray or purplish-brown or greenish margin growth darkening to black Sharp, pungent smell Strong, pungent bitter- ODOR Slight cherry odor None when broken almond odor BUDS Cone shaped, slender – Football-shaped with a SHAPE Ovate, -

The Genus Fusicladium (Hyphomycetes) in Poland
ACTA MYCOLOGICA Dedicated to Professor Alina Skirgiełło Vol. 41 (2): 285-298 on the occasion of her ninety-fifth birthday 2006 The genus Fusicladium (Hyphomycetes) in Poland MAŁGORZATA RUSZKIEWICZ-MICHALSKA and EWA POŁEĆ 1 Department of Algology and Mycology, University of Łódź, Banacha 12/16, PL-90-237 Łódź [email protected]; [email protected] Ruszkiewicz-Michalska M., Połeć E.: The genus Fusicladium (Hyphomycetes) in Poland. Acta Mycol. 41 (2): 285-298, 2006. The paper presents new and historical data on the genus Fusicladium verified on the base of the recently published critical monograph. Fifteen species recorded in Poland under the name Fusicladium and synonymous Pollaccia and Spilocaea are reported; 5 are documented by authors’ materials from Central Poland while the other taxa are supported with literature data only, including three species belonging currently to Fusicladiella and Passalora. Three species, reported here for the first time in Poland: Fusicladium convolvularum Ondřej, F. scribnerianum (Cavara) M. B. Ellis and F. virgaureae Ondřej, are known from a few localities in the world. All the species are provided with the distribution maps and the newly reported ones are illustrated with ink drawings. Key words: parasitic fungi, anamorphic fungi, Deuteromycotina, distribution, Poland INTRODUCTION Worldwide 57 fungal taxa belong to the anamorphic genus Fusicladium Bonord. em. Schubert, Ritschel et U. Braun. They are phytopathologically relevant patho- gens, causing leaf spots, necroses, scab diseases as well as leaf and fruit deformations of members of at least 52 angiospermous plant genera (Schubert, Ritschel, Braun 2003). The fungi are host specific, mostly confined to a single host genus or allied host genera in a single family, e.g. -

<I>Tothia Fuscella</I>
ISSN (print) 0093-4666 © 2011. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/118.203 Volume 118, pp. 203–211 October–December 2011 Epitypification, morphology, and phylogeny of Tothia fuscella Haixia Wu1, Walter M. Jaklitsch2, Hermann Voglmayr2 & Kevin D. Hyde1, 3, 4* 1 International Fungal Research and Development Centre, Key Laboratory of Resource Insect Cultivation & Utilization, State Forestry Administration, The Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, 650224, PR China 2 Department of Systematic and Evolutionary Botany, Faculty Centre of Biodiversity, University of Vienna, Rennweg 14, A-1030 Wien, Austria 3 School of Science, Mae Fah Luang University, Tasud, Muang, Chiang Rai 57100, Thailand 4 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 11442, Saudi Arabia *Correspondence to: [email protected] Abstract — The holotype of Tothia fuscella has been re-examined and is re-described and illustrated. An identical fresh specimen from Austria is used to designate an epitype with herbarium material and a living culture. Sequence analyses show T. fuscella to be most closely related to Venturiaceae and not Microthyriaceae, to which it was previously referred. Key words — Dothideomycetes, molecular phylogeny, taxonomy Introduction We have been re-describing and illustrating the generic types of Dothideomycetes (Zhang et al. 2008, 2009, Wu et al. 2010, 2011, Li et al. 2011) and have tried where possible to obtain fresh specimens for epitypification and use molecular analyses to provide a natural classification. Our previous studies of genera in the Microthyriaceae, a poorly known family within the Dothideomycetes, have resulted in several advances (Wu et al. -

High Diversity and Morphological Convergence Among Melanised Fungi from Rock Formations in the Central Mountain System of Spain
Persoonia 21, 2008: 93–110 www.persoonia.org RESEARCH ARTICLE doi:10.3767/003158508X371379 High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain C. Ruibal1, G. Platas2, G.F. Bills2 Key words Abstract Melanised fungi were isolated from rock surfaces in the Central Mountain System of Spain. Two hundred sixty six isolates were recovered from four geologically and topographically distinct sites. Microsatellite-primed biodiversity PCR techniques were used to group isolates into genotypes assumed to represent species. One hundred and sixty black fungi three genotypes were characterised from the four sites. Only five genotypes were common to two or more sites. Capnodiales Morphological and molecular data were used to characterise and identify representative strains, but morphology Chaetothyriales rarely provided a definitive identification due to the scarce differentiation of the fungal structures or the apparent Dothideomycetes novelty of the isolates. Vegetative states of fungi prevailed in culture and in many cases could not be reliably dis- extremotolerance tinguished without sequence data. Morphological characters that were widespread among the isolates included scarce micronematous conidial states, endoconidia, mycelia with dark olive-green or black hyphae, and mycelia with torulose, isodiametric or moniliform hyphae whose cells develop one or more transverse and/or oblique septa. In many of the strains, mature hyphae disarticulated, suggesting asexual reproduction by a thallic micronematous conidiogenesis or by simple fragmentation. Sequencing of the internal transcribed spacers (ITS1, ITS2) and 5.8S rDNA gene were employed to investigate the phylogenetic affinities of the isolates. According to ITS sequence alignments, the majority of the isolates could be grouped among four main orders of Pezizomycotina: Pleosporales, Dothideales, Capnodiales, and Chaetothyriales. -

Fungal Planet Description Sheets: 400–468
Persoonia 36, 2016: 316– 458 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158516X692185 Fungal Planet description sheets: 400–468 P.W. Crous1,2, M.J. Wingfield3, D.M. Richardson4, J.J. Le Roux4, D. Strasberg5, J. Edwards6, F. Roets7, V. Hubka8, P.W.J. Taylor9, M. Heykoop10, M.P. Martín11, G. Moreno10, D.A. Sutton12, N.P. Wiederhold12, C.W. Barnes13, J.R. Carlavilla10, J. Gené14, A. Giraldo1,2, V. Guarnaccia1, J. Guarro14, M. Hernández-Restrepo1,2, M. Kolařík15, J.L. Manjón10, I.G. Pascoe6, E.S. Popov16, M. Sandoval-Denis14, J.H.C. Woudenberg1, K. Acharya17, A.V. Alexandrova18, P. Alvarado19, R.N. Barbosa20, I.G. Baseia21, R.A. Blanchette22, T. Boekhout3, T.I. Burgess23, J.F. Cano-Lira14, A. Čmoková8, R.A. Dimitrov24, M.Yu. Dyakov18, M. Dueñas11, A.K. Dutta17, F. Esteve- Raventós10, A.G. Fedosova16, J. Fournier25, P. Gamboa26, D.E. Gouliamova27, T. Grebenc28, M. Groenewald1, B. Hanse29, G.E.St.J. Hardy23, B.W. Held22, Ž. Jurjević30, T. Kaewgrajang31, K.P.D. Latha32, L. Lombard1, J.J. Luangsa-ard33, P. Lysková34, N. Mallátová35, P. Manimohan32, A.N. Miller36, M. Mirabolfathy37, O.V. Morozova16, M. Obodai38, N.T. Oliveira20, M.E. Ordóñez39, E.C. Otto22, S. Paloi17, S.W. Peterson40, C. Phosri41, J. Roux3, W.A. Salazar 39, A. Sánchez10, G.A. Sarria42, H.-D. Shin43, B.D.B. Silva21, G.A. Silva20, M.Th. Smith1, C.M. Souza-Motta44, A.M. Stchigel14, M.M. Stoilova-Disheva27, M.A. Sulzbacher 45, M.T. Telleria11, C. Toapanta46, J.M. Traba47, N. -

<I>Acrocordiella</I>
Persoonia 37, 2016: 82–105 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158516X690475 Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria W.M. Jaklitsch1,2, A. Gardiennet3, H. Voglmayr2 Key words Abstract Fresh material, type studies and molecular phylogeny were used to clarify phylogenetic relationships of the nine genera Acrocordiella, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Ascomycota Requienella, Seiridium and Strickeria. At first sight, some of these genera do not seem to have much in com- Dothideomycetes mon, but all were found to belong to the Xylariales, based on their generic types. Thus, the most peculiar finding new genus is the phylogenetic affinity of the genera Acrocordiella, Requienella and Strickeria, which had been classified in phylogenetic analysis the Dothideomycetes or Eurotiomycetes, to the Xylariales. Acrocordiella and Requienella are closely related but pyrenomycetes distinct genera of the Requienellaceae. Although their ascospores are similar to those of Lepteutypa, phylogenetic Pyrenulales analyses do not reveal a particularly close relationship. The generic type of Lepteutypa, L. fuckelii, belongs to the Sordariomycetes Amphisphaeriaceae. Lepteutypa sambuci is newly described. Hymenopleella is recognised as phylogenetically Xylariales distinct from Lepteutypa, and Hymenopleella hippophaëicola is proposed as new name for its generic type, Spha eria (= Lepteutypa) hippophaës. Clypeosphaeria uniseptata is combined in Lepteutypa. No asexual morphs have been detected in species of Lepteutypa. Pseudomassaria fallax, unrelated to the generic type, P. chondrospora, is transferred to the new genus Basiseptospora, the genus Pseudapiospora is revived for P. corni, and Pseudomas saria carolinensis is combined in Beltraniella (Beltraniaceae).