Inductive Heating Using a High-Magnetic-Field Pulse to Initiate Chemical Reactions to Generate Composite Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
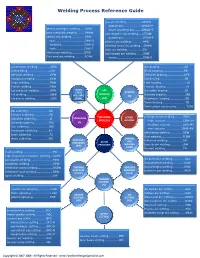
Welding Process Reference Guide
Welding Process Reference Guide gas arc welding…………………..GMAW -pulsed arc…………….……….GMAW-P atomic hydrogen welding……..AHW -short circuiting arc………..GMAW-S bare metal arc welding…………BMAW gas tungsten arc welding…….GTAW carbon arc welding……………….CAW -pulsed arc……………………….GTAW-P -gas……………………………………CAW-G plasma arc welding……………..PAW -shielded……………………………CAW-S shielded metal arc welding….SMAW -twin………………………………….CAW-T stud arc welding………………….SW electrogas welding……………….EGW submerged arc welding……….SAW Flux cord arc welding…………..FCAW -series………………………..…….SAW-S coextrusion welding……………...CEW Arc brazing……………………………..AB cold welding…………………………..CW Block brazing………………………….BB diffusion welding……………………DFW Diffusion brazing…………………….DFB explosion welding………………….EXW Dip brazing……………………………..DB forge welding…………………………FOW Flow brazing…………………………….FLB friction welding………………………FRW Furnace brazing……………………… FB hot pressure welding…………….HPW SOLID ARC Induction brazing…………………….IB STATE BRAZING WELDING Infrared brazing……………………….IRB roll welding…………………………….ROW WELDING (8) ultrasonic welding………………….USW (SSW) (AW) Resistance brazing…………………..RB Torch brazing……………………………TB Twin carbon arc brazing…………..TCAB dip soldering…………………………DS furnace soldering………………….FS WELDING OTHER electron beam welding………….EBW induction soldering……………….IS SOLDERING PROCESS WELDNG -high vacuum…………………….EBW-HV infrared soldering…………………IRS (S) -medium vacuum………………EBW-MV iron soldering……………………….INS -non-vacuum…………………….EBW-NV resistance soldering…………….RS electroslag welding……………….ESW torch soldering……………………..TS -

Optimal Design of High-Frequency Induction Heating Apparatus for Wafer Cleaning Equipment Using Superheated Steam
energies Article Optimal Design of High-Frequency Induction Heating Apparatus for Wafer Cleaning Equipment Using Superheated Steam Sang Min Park 1 , Eunsu Jang 2, Joon Sung Park 1 , Jin-Hong Kim 1, Jun-Hyuk Choi 1 and Byoung Kuk Lee 2,* 1 Intelligent Mechatronics Research Center, Korea Electronics Technology Institute (KETI), Bucheon 14502, Korea; [email protected] (S.M.P.); [email protected] (J.S.P.); [email protected] (J.-H.K.); [email protected] (J.-H.C.) 2 Department of Electrical and Computer Engineering, Sungkyunkwan University (SKKU), Suwon 16419, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-31-299-4581 Received: 19 October 2020; Accepted: 22 November 2020; Published: 25 November 2020 Abstract: In this study, wafer cleaning equipment was designed and fabricated using the induction heating (IH) method and a short-time superheated steam (SHS) generation process. To prevent problems arising from the presence of particulate matter in the fluid flow region, pure grade 2 titanium (Ti) R50400 was used in the wafer cleaning equipment for heating objects via induction. The Ti load was designed and manufactured with a specific shape, along with the resonant network, to efficiently generate high-temperature steam by increasing the residence time of the fluid in the heating object. The IH performance of various shapes of heating objects made of Ti was analyzed and the results were compared. In addition, the heat capacity required to generate SHS was mathematically calculated and analyzed. The SHS heating performance was verified by conducting experiments using the designed 2.2 kW wafer cleaning equipment. -

Estimation of Weld Quality in High-Frequency Electric Resistance Welding with Image Processing
Kim March 2007 LAYOUT:Layout 1 2/8/07 2:30 PM Page 71 WELDING RESEARCH Estimation of Weld Quality in High-Frequency Electric Resistance Welding with Image Processing An image analysis algorithm can estimate the weld quality by using the weld image data during high-frequency electric resistance welding BY D. KIM, T. KIM, Y. W. PARK, K. SUNG, M. KANG, C. KIM, C. LEE, AND S. RHEE ABSTRACT. In high-frequency electric duction welding method, an induction coil surface is compressed by the squeeze resistance welding (HF-ERW), the weld is used in inducing high-frequency current roller, excreting oxided material from the quality is determined by the welding phe- to generate heat. In high-frequency resis- melting surface to generate a weld. The nomenon in the welding spot proximity. tance welding, on the contrary, a contac- molten part of the pipe generated by the Methods to eliminate or minimize defects, tor is applied to the workpiece to directly resistance heat contains oxided material therefore, include real-time monitoring of provide the current (Ref. 1). High- on account of exposure to the air. There- the weld quality and problem solving as frequency induction welding is primarily fore, if the oxided material is not com- problems arise. It is possible to estimate used in joining small-diameter steel pipes, pletely excreted during compression, and weld quality qualitatively by using the weld while HF-ERW is used in joining large- oxided material remains in the welding image data during HF-ERW. A method to diameter steel pipes. part as an impurity, the weld quality be- predict the weld quality using image pro- The characteristic of HF-ERW is the re- comes degraded. -

Design of a Wireless Power Transfer System Using Inductive Coupling and MATLAB Programming Apoorva.P1 Deeksha.K.S2 Pavithra.N3 Student, Dept
International Journal on Recent and Innovation Trends in Computing and Communication ISSN: 2321-8169 Volume: 3 Issue: 6 3817 - 3825 _______________________________________________________________________________________________ Design of a Wireless Power Transfer System using Inductive Coupling and MATLAB programming Apoorva.P1 Deeksha.K.S2 Pavithra.N3 Student, Dept. Of EEE, Student, Dept. Of EEE Student, Dept. Of EEE, Dr. T. Thimmaiah Institute of Dr. T. Thimmaiah Institute of Dr. T. Thimmaiah Institute of Technology Technology Technology K.G.F,India K.G.F, India K.G.F, India [email protected] [email protected] [email protected] Vijayalakshmi.M.N4 Somashekar.B5 David Livingston.D6 Student, Dept. Of EEE Asst Professor Dept. Of EEE, Asst Professor Dept. Of EEE, Dr. T. Thimmaiah Institute of Dr. T. Thimmaiah Institute of Dr. T. Thimmaiah Institute of Technology Technology Technology K.G.F, India K.G.F, India K.G.F, India [email protected] [email protected] [email protected] Abstract—Wireless power transfer (WPT) is the propagation of electrical energy from a power source to an electrical load without the use of interconnecting wires. It is becoming very popular in recent applications. Wireless transmission is useful in cases where interconnecting wires are difficult, hazardous, or non-existent. Wireless power transfer is becoming popular for induction heating, charging of consumer electronics (electric toothbrush, charger), biomedical implants, radio frequency identification (RFID), contact-less smart cards, and even for transmission of electrical energy from space to earth. In the design of the coils, the parameters of coils are obtained by using the basic calculations and measurements. -

Induction Heating Principles PRESENTATION
Induction Heating Principles PRESENTATION www.ceia-power.com This document is property of CEIA which reserves all rights. Total or partial copying, modification and translation is forbidden FC040K0068V1000UK Main Applications of Induction Heating ¾ Hard (Silver) Brazing ¾ Tin Soldering ¾ Heat Treatment (Hardening, Annealing, Tempering, …) ¾ Melting Applications (ferrous and non ferrous metal) ¾ Forging This document is property of CEIA which reserves all rights. Total or partial copying, modification and translation is forbidden FC040K0068V1000UK Examples of induction heating applications This document is property of CEIA which reserves all rights. Total or partial copying, modification and translation is forbidden FC040K0068V1000UK Advantages of Induction Reduced Heating Time Localized Heating Efficient Energy Consumption Heating Process Controllable and Repeatable Improved Product Quality Safety for User Improving of the working condition This document is property of CEIA which reserves all rights. Total or partial copying, modification and translation is forbidden FC040K0068V1000UK Basics of Induction INDUCTIVE HEATING is based on the supply of energy by means of electromagnetic induction. A coil, suitably dimensioned, placed close to the metal parts to be heated, conducting high or medium frequency alternated current, induces on the work piece currents (eddy currents) whose intensity can be controlled and modulated. This document is property of CEIA which reserves all rights. Total or partial copying, modification and translation is forbidden FC040K0068V1000UK Basics of induction The heating occurs without physical contact, it involves only the metal parts to be treated and it is characterized by a high efficiency transfer without loss of heat. The depth of penetration of the generated currents is directly correlated to the working frequency of the generator used; higher it is, much more the induced currents concentrate on the surface. -

Magnetic Flux Controllers in Induction Heating and Melting
ASM Handbook, Volume 4C, Induction Heating and Heat Treatment Copyright # 2014 ASM InternationalW V. Rudnev and G.E. Totten, editors All rights reserved www.asminternational.org Magnetic Flux Controllers in Induction Heating and Melting Robert Goldstein, Fluxtrol, Inc. MAGNETIC FLUX CONTROLLERS are workpiece. For both cases, there are three closed is applied, it strongly reduces the reluctance of materials other than the copper coil that are used loops: flow of current in the coil, flow of mag- the back path for the magnetic flux (Ref 3). All in induction systems to alter the flow of the mag- netic flux, and flow of current in the workpiece. induction heating systems can be described in netic field. Magnetic flux controllers used in In most cases, the difference between induc- this way. power supplying components are not considered tion heating applications and transformers is that The benefits of a magnetic flux concentrator in this article. the magnetic circuit is open. The magnetic field on the electrical parameters for a given applica- Magnetic flux controllers have been in exis- path includes not only the area with the control- tion depends on the ratio of the reluctance of tence since the development of the induction ler, but also the workpiece surface layer and the the back path for magnetic flux to the overall technique. Michael Faraday used two coils of air between the surface and controller, which reluctance in the system. It is also possible to wire wrapped around an iron core in his experi- cannot be changed. Therefore, the reluctance of break down basic system components into sub- ments that led to Faraday’s law of electromagnetic the magnetic path only partially depends on the components to determine the most economical induction, which states that the electromotive magnetic permeability of the controller (Ref 3). -

Handbook of Induction Heating Theoretical Background
This article was downloaded by: 10.3.98.104 On: 28 Sep 2021 Access details: subscription number Publisher: CRC Press Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: 5 Howick Place, London SW1P 1WG, UK Handbook of Induction Heating Valery Rudnev, Don Loveless, Raymond L. Cook Theoretical Background Publication details https://www.routledgehandbooks.com/doi/10.1201/9781315117485-3 Valery Rudnev, Don Loveless, Raymond L. Cook Published online on: 11 Jul 2017 How to cite :- Valery Rudnev, Don Loveless, Raymond L. Cook. 11 Jul 2017, Theoretical Background from: Handbook of Induction Heating CRC Press Accessed on: 28 Sep 2021 https://www.routledgehandbooks.com/doi/10.1201/9781315117485-3 PLEASE SCROLL DOWN FOR DOCUMENT Full terms and conditions of use: https://www.routledgehandbooks.com/legal-notices/terms This Document PDF may be used for research, teaching and private study purposes. Any substantial or systematic reproductions, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The publisher shall not be liable for an loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. 3 Theoretical Background Induction heating (IH) is a multiphysical phenomenon comprising a complex interac- tion of electromagnetic, heat transfer, metallurgical phenomena, and circuit analysis that are tightly interrelated and highly nonlinear because the physical properties of materi- als depend on magnetic field intensity, temperature, and microstructure. -

Induction Heating: Fundamentals
30/10/17 LEP ELECTROMAGNETIC PROCESSING OF MATERIALS TECNOLGIE DEI PROCESSI ELETTROTERMICI Induction Heating: fundamentals Fabrizio Dughiero 2017-2018 Induction heating fundamentals May 28-30, 2014 1 30/10/17 Summary 1. Induction heating physical principles 2. Characteristics of the induction heating process • Physical parameters that affect induction heating 3. The skin effect: • What parameters modify the skin effect? • Change of skin effect during the heating 4. Examples: • Heating of a magnetic billet • Choosing the frequency appropriate to the workpiece • Coil thickness as a function of frequency 5. Proximity effect, ring effect, flux concentrators effect 1. Induction heating physical principles May 28-30, 2014 2 30/10/17 Induction heating physical principles Characteristics of induction heating • High temperature in the workpiece (in most cases). • High power density for a short heating time (in many applications). • High frequency (in many applications). • Thermal sources are inside the workpiece. Induction heating physical principles Induction heating: fundamental laws ? They state: A. Maxwell’s equations • how the electromagnetic (e.m.) field is generated rd 3 Maxwell’s equation or • how the e.m. field propagates and is Faraday-Neumann-Lenz’s law distributed in the space 4th Maxwell’s equation or • how the e.m. field interacts with the Ampere’s law charged particles. • they state what is the (approximate) B. Constitutive relations response of a specific material to an for materials external field or force. ? Ohm’s law Magnetic -

Induction Heat Treatment
a ...... ...... ....... ..... ...... .... ... ..... ...... .... ... ..... ...... ....... ..... ...... ....,...... ..... ...... .... ... ..... ...... ....... ..... ...... ....... ..... ...... .... ... ....I ...... ....... ..... ...... ....... ..... ...... ....... ..... ...... ....... ..... ...... .... ... ..... ...... .... ... ...... .... ... ...... .... ... ...... ....... ...... .... ... ...... ,....... ... ...... .... ... .....I .... .... ..... ... ... .....(... ....... ... ..... ... ........ ... ... ..... ...... i iG -i Publishedby the EPRl Center for MaterialsFabrication Vol. 2, No. 2, 1985Reprinted March, 1990 Using Induction Heat speed and selective heating capabil- Vol. 2, No. 1. Advantages specific to Treatment to Obtain ity, to produce quality parts cost induction heat treating are: Special Properties effectively. It will answer such ques- Speed - In heat treatment, the Cost Effectively tions as: What are the advantages higher heating rates play a central of induction heat treatment? What role in designing rapid, high- Heat treatment is often one of the heat treatments can I conduct with temperature heat treating most important stages of metal induction? What are some typical processes. Induction heat treating of processing because it determines parts and materials that are induc- steel may take as little as the final properties that enable tion heat treated? What properties 10 percent or less of the time cqmponents to perform under such can I obtain with induction heat required for furnace treatment. demanding service conditions -
Induction Heating Work Coils
Induction Heating Work Coils The work coil, also known as the inductor, is the component in the induction heating system that defines how effective and how efficiently the work piece is heated. Work coils range in complexity from a simple helical (or solenoid) wound coil consisting of a number of turns of copper tube wound around a mandrel to a coil precision machined from solid copper and brazed together. The work coil is used to transfer the energy from the induction heating power supply and workhead to the work piece by generating an alternating electromagnetic field. The electromagnetic field generates a current that flows in the work piece as a mirror image to the current flowing in the work coil. As the current flows through the resistivity of the work piece it generates the heat within the work piece from I²R losses. A second heating principle, hysteretic heating is also in effect when the work piece is a magnetic material such as carbon steel. Energy is generated within the work piece by the alternating magnetic field changing the magnetic polarity within the work piece. Hysteretic heating occurs in the work piece only up to the Curie temperature (750° C for steel) when the material’s magnetic permeability decreases to 1. Work Coil Basics A current flowing in a conductor creates a magnetic field. An alternating current creates an alternating field which produces an alternating current in a second conductor (the work piece). The current in the work piece is proportional to the field strength. The transformer effect where the amount of current induced in the work piece is proportional to the number of turns on the coil and is generated as a mirror image of the work coil. -

Introduction
Elements of Induction Heating Copyright © 1988 ASM International® Stanley Zinn, Lee Semiatin, p 1-8 All rights reserved. DOI: 10.1361/eoih1988p001 www.asminternational.org Chapter 1 Introduction Electromagnetic induction, or simply "induction," is a method of heating electrically conductive materials such as metals. It is commonly used in process heating prior to metalworking, and in heat treating, welding, and melting (Table 1.1). This technique also lends itself to various other applications involving packaging and curing. The number of industrial and consumer items which undergo induction heating during some stage of their production is very large and rapidly expanding. As its name implies, induction heating relies on electrical currents that are induced internally in the material to be heated-i.e., the workpiece. These Table 1,1. Induction heating applications and typical products Preheating prior to metalworking Heat treating Welding Melting Forging Surface Hardening, Seam Welding Air Melting of Steels Gears Tempering Oil-country Ingots Shafts Gears tubular Billets Hand tools Shafts products Castings Ordnance Valves Refrigeration Vacuum Induction Machine tools tubing Melting Extrusion Hand tools Line pipe Structural Ingots members Through Hardening, Billets Shafts Tempering Castings Structural "Clean" steels Heading members Nickel-base Bolts Spring steel superalloys Other fasteners Chain links Titanium alloys Rolling Slab Annealing Aluminum strip Sheet (can, ap- pliance, and Steel strip automotive industries) 2 Elements of Induction Heating: Design, Control, and Applications so-called eddy currents dissipate energy and bring about heating. The basic components of an induction heating system are an induction coil, an alter- nating-current (ac) power supply, and the workpiece itself. -

Specifics of Induction Hardening of Powder Metallurgy (P/M) Components
INDUCTION THOUGHTS INDUCTIONINDUCTION THOUGHTSTHOUGHTS Dr. Valery Rudnev, known as “Professor Induction”, discusses in the heat processing diffe- rent aspects of induction heating, novel theoretical and practical knowledge related to dif- ferent heat treating technologies accumulated in the North America and around the globe. Specifics of induction hardening of powder metallurgy (P/M) components uring the last decade, the use of advanced powder metallurgy type of heat treatment. However, differences in electromagnetic D(P/M) materials in several industries has been expanded at properties are specific for processing those materials applying an impressive pace further penetrating markets of demanding electromagnetic induction. Table 1 summarizes the effect of applications; however, the biggest market for ferrous P/M com- density reduction and porosity increase on IH. ponents remains to be the transportation industry, particularly The relative magnetic permeability μr of P/M materials is consid- the automotive and agricultural sectors. An ability to manufacture erably lower than the μr of the corresponding wrought steels, while net-shape complex geometries offering competitive performance their electrical resistivities ρ are greater to some extent. Both factors at an economical cost is an attractive feature of P/M parts (e. g. gears, lead to noticeably larger current penetration depth (δ) during the timing sprockets, cams, splined hubs, shafts, shock absorbers etc.) heating cycle, affecting not only heat generation and temperature [1]. Density and porosity in the sintered compact are major factors profiles but also the magnitude and distribution of transient and affecting strength and hardenability of ferrous P/M materials; both residual stresses as well as coil electrical parameters.