Ideal Gases at Low Gas Densities, All Gases Can Be Treated As Ideal Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biochemical Thermodynamics
Biochemical Thermodynamics Biochemical Thermodynamics By Juan S. Jiménez Biochemical Thermodynamics By Juan S. Jiménez This book first published 2020 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2020 by Juan S. Jiménez All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-5359-0 ISBN (13): 978-1-5275-5359-0 To the memory of Brígida and Francisco Jiménez CONTENTS PREFACE ..................................................................................................... x CHAPTER 1 .................................................................................................. 1 INTRODUCTION 1.1 The Atomic Theory of John Dalton and the Hypothesis of Amedeo Avogadro 1.2 The Mole and Avogadro’s Number 1.3 The ideal gas model 1.4 The Periodic Table and Initial Atomic Theories 1.5 The Hydrogen Atom and the Schrödinger Equation 1.6 Atomic Structure 1.7 Molecules CHAPTER 2 ................................................................................................ 35 ENTROPY 2.1 Systems, Properties and States 2.2 The First Law of Thermodynamics 2.3 Enthalpy 2.4 Reversible changes 2.5 The Second Law of Thermodynamics 2.6 A Particle in a One-dimensional Box 2.7 Quantum States 2.8 The Boltzmann Equation CHAPTER 3 ................................................................................................ 63 THE CHEMICAL EQUILIBRIUM 3.1 The Gibbs Function 3.2 The Chemical Potential 3.3 Chemical Equilibrium 3.4 Model Systems 3.5 The Equilibrium Constant for Chemical Reactions between Gases. -

Avogadro Number, Boltzmann Constant, Planck Constant, Information Theory, Comparative and Relative Uncertainties
American Journal of Computational and Applied Mathematics 2018, 8(5): 93-102 DOI: 10.5923/j.ajcam.20180805.02 h, k, NA: Evaluating the Relative Uncertainty of Measurement Boris Menin Mechanical & Refrigeration Consultation Expert, Beer-Sheba, Israel Abstract For verification of the measurement accuracy of fundamental physical constants, the CODATA unique technique is used. This procedure is a complex process based on a careful discussion of the input data, and the justification and construction of tables of values sufficient for the direct use of the relative uncertainty are conducted using modern advanced statistical methods and powerful computers. However, at every stage of data processing, researchers must rely on common sense, that is, an expert conclusion. In this article, the author proposes a theoretical and informational grounded justification for calculating the relative uncertainty by using the comparative uncertainty. A detailed description of the data and the processing procedures do not require considerable time. Examples of measurements results of three fundamental constants are analysed, applying information-oriented approach. Comparison of the achieved relative and comparative uncertainties is introduced and discussed. Keywords Avogadro number, Boltzmann constant, Planck constant, Information theory, Comparative and relative uncertainties theories of physics can be proved by carefully studying the 1. Introduction numerical values of these constants, determined from different experiments in different fields of physics. It is expected that in 2018 that the International System of One needs to note the main feature of the CODATA Units (SI) will be redefined on the basis of certain values of technique in determining the relative uncertainty of one some fundamental constants [1]. -

Amedeo Avogadro
Amedeo Avogadro ALSO LISTED IN Physicists FAMOUS AS Chemist and Physicist NATIONALITY Italian Famous Italian Men RELIGION Roman Catholic BORN ON 09 August 1776 AD Famous 9th August Birthdays ZODIAC SIGN Leo Leo Men BORN IN Turin, Italy DIED ON 09 July 1856 AD PLACE OF DEATH Turin, Italy FATHER Filippo Avogadro MOTHER Anna Maria Vercellone SPOUSE: Felicita Mazzé Lorenzo Romano Amedeo Carlo Avogadro de Quaregna e di Cerreto, more popularly known as Amedeo Avogadro was born on August 9, 1776, in Turin, Italy. He was a gifted physicist and chemist who proposed the molecular theory, which is more popularly known as ‘Avogadro’s Law’. Although he earned a doctorate in ecclesiastical law, he developed a passion for studying mathematics and physics. He then gave up his career in law and pursued a career teaching natural physics at the Royal College of Vercelli. Years later, he was offered the chair of mathematical physics at the University of Turin. Avogadro conducted experiments in both physics and chemistry using mathematics as a basis for his findings. His hypothesis, known as the ‘Avogadro’s Law’ is recognized all over the world. He also published many works during his lifetime. The number 6.02214199 x 10^23 is named as Avogadro’s number to honor him for his contribution in molecular theory. Read on to know more about this great physicist and chemist. Read more at http://www.thefamouspeople.com/profiles/amadeo-avogadro- 532.php#09s5lE6QjEOdfSro.99 Career After studying philosophy in 1789, Amedeo Avogadro graduated in jurisprudence in 1792 and earned his doctorate in ecclesiastical law in 1796. -

Chemistry in Italy During Late 18Th and 19Th Centuries
CHEMISTRY IN ITALY DURING LATE 18TH AND 19TH CENTURIES Ignazio Renato Bellobono, CSci, CChem, FRSC LASA, Department of Physics, University of Milan. e-mail add ress : i.bell obon o@ti scali.it LASA, Dept.Dept. ofPhysics, Physics, University of Milan The birth of Electrochemistry Luigi Galvani, Alessandro Volta, and Luigi Valentino Brugnatelli From Chemistry to Radiochemistry The birth of Chemistry and Periodic Table Amedeo Avogadro and Stanislao Cannizzaro Contributions to Organic Chemistry LASA, Dept.Dept. ofPhysics, Physics, University of Milan 1737 At the Faculty of Medicine of the Bologna University, the first chair of Chemistry is establishedestablished,,andandassigned to Jacopo Bartolomeo BECCARI (1692-1766). He studied phosphorescence and the action of light on silver halides 1776 In some marshes of the Lago Maggiore, near AngeraAngera,, Alessandro VOLTA ((17451745--18271827),),hi gh school teacher of physics in Como, individuates a flammable gas, which he calls aria infiammabile. Methane is thus discovereddiscovered.. Two years laterlater,,heheis assignedassigned,,asas professor of experimental phihysicscs,,toto the UiUniversi ty of PiPavia LASA, DtDept. of PhPhys icscs,, University of Milan 1778 In aletter a letter to Horace Bénédict de Saussure, aaSwissSwiss naturalist, VOLTA introduces, beneath that of electrical capacitycapacity,, the fundamental concept of tensione elettrica (electrical tension), exactly the name that CITCE recommended for the difference of potential in an electrochemical cell. 17901790--17911791 VOLTA anticipatesanticipates,,bybyabout 10 yearsyears,,thethe GAYGAY--LUSSACLUSSAC linear de ppyendency of gas volume on tem pp,erature, at constant pressurepressure,,andandafew a fewyears later ((17951795)) anticipatesanticipates,,byby about 6years 6 years,,thethe soso--calledcalled John Dalton’s rules ((18011801))ononvapour pressure LASA, Dept.Dept. -

X-Ray Crystal Density Method to Determine the Avogadro and Planck Constants
X-ray Crystal Density Method to Determine the Avogadro and Planck Constants Horst Bettin Physikalisch-Technische Bundesanstalt Braunschweig, Germany Proposed New Definition of the Kilogram The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 040 x 10 -34 when expressed in the unit J s, which is equal to kg m2 s-1, where the metre and the second c ∆ν are defined in terms of and Cs . *) X represents one or more digits to be added at the time the new definition is finally adopted. α 2 - = M (e )c NAh 2 R∞ N h −10 A = 3.990 312 7110(18) × 10 Js/mol, Amedeo Avogadro Max Planck with relative uncertainty of 0.45 × 10 -9 (1776-1856) (1858-1947) Page 2 of 19 Avogadro Constant Definition of Avogadro constant NA • Number of molecules per mol • 6.022... x 10 23 mol -1 Amedeo Avogadro Current definition of mol (1776-1856) • Number “entities” like 12 C atoms in 12 g • i. e. 6.022... x 10 23 12 C atoms have a mass of 12 g 12 12 g/mol = NA m( C ) Faraday constant F = NA e (e: elementary charge) Molar gas constant R = NA k (k: Boltzmann constant) Page 3 of 19 Counting Atoms: XRCD Method 3 Use of a silicon crystal! 1. Volume a0 of the unit cell 3 2. Volume of an atom: a0 /8 3. Volume V of a sphere 4. Number N of the atoms 8 V8 VM N = = ⋅ mol A N 3 3 a0 a0msphere Page 4 of 19 Lattice parameter measurement (INRIM) d220 (2011)=192014712.67(67) am d220 (2014)=192014711.98(34) am -9 ur(2014) = 1.8 x 10 Page 5 of 19 Lattice parameter set-up at PTB S M1 M M2 AL AA OH Page 6 of 19 Sphere Interferometer of PTB mK-temperature stabilisation Camera 1 Camera 2 Fizeau- Fizeau- Collimator Objective 1 Objective 2 Diode laser Page 7 of 19 Diameter results (2014) Relative Mean apparent Sphere Lab. -

2 Weighing and Preparation of Aqueous Solutions
2 WEIGHING AND PREPARATION OF AQUEOUS SOLUTIONS Theory In chemistry, a solution is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is dissolved in another substance, known as a solvent. An aqueous solution is a solution in which the solvent is water. Concentration is the measure of how much of a given substance (solute) there is mixed with another substance (solvent). This can apply to any sort of chemical mixture, but most frequently the concept is limited to homogeneous solutions, where it refers to the amount of solute in a solution. For scientific or technical applications, concentration is expressed in a quantitative way. There are a number of different ways to quantitatively express concentration; the most common are listed below. They are based on mass or volume or both. Depending on what they are based on it is not always trivial to convert one measure to the other, because knowledge of the density might be needed to do so. At times this information may not be available, particularly if the temperature varies. Mass can be determined at a precision of ~ 0.1 mg on a routine basis with an analytical balance. Both solids and liquids are easily quantified by weighing. Some units of concentration - particularly the most popular one (molarity) - require to express the mass of substance in the moles. The mol is the SI basic unit that measures an amount of substance. „One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the −1 fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol and is called the Avogadro number.“ The previous definition was based on the number of carbon atoms in 12 g of material and was the following; a mol is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram (or 12 g) of carbon-12 (12C), where the carbon – 12 atoms are unbound, at rest and in their ground state. -

Topic720 Composition: Mole Fraction: Molality: Concentration a Solution Comprises at Least Two Different Chemical Substances
Topic720 Composition: Mole Fraction: Molality: Concentration A solution comprises at least two different chemical substances where at least one substance is in vast molar excess. The term ‘solution’ is used to describe both solids and liquids. Nevertheless the term ‘solution’ in the absence of the word ‘solid’ refers to a liquid. Chemists are particularly expert at identifying the number and chemical formulae of chemical substances present in a given closed system. Here we explore how the chemical composition of a given system is expressed. We consider a simple system prepared using water()l and urea(s) at ambient temperature and pressure. We designate water as chemical substance 1 and urea as chemical substance j, so that the closed system contains an aqueous solution. The amounts of the two substances are given by n1 = = ()wM11 and nj ()wMjj where w1 and wj are masses; M1 and Mj are the molar masses of the two chemical substances. In these terms, n1 and nj are extensive variables. = ⋅ + ⋅ Mass of solution, w n1 M1 n j M j (a) = ⋅ Mass of solvent, w1 n1 M1 (b) = -1 For water, M1 0.018 kg mol . However in reviewing the properties of solutions, chemists prefer intensive composition variables. Mole Fraction The mole fractions of the two substances x1 and xj are given by the following two equations: =+ =+ xnnn111()j xnnnjj()1 j (c) += Here x1 x j 10. In general terms for a system comprising i - chemical substances, the mole fraction of substance k is given by equation (d). ji= = xnkk/ ∑ n j (d) j=1 ji= = Hence ∑ x j 10. -

Avogadro's Constant
Avogadro's Constant Nancy Eisenmenger Question Artem, 8th grade How \Avogadro constant" was invented and how scientists calculated it for the first time? Answer What is Avogadro's Constant? • Avogadros constant, NA: the number of particles in a mole • mole: The number of Carbon-12 atoms in 0.012 kg of Carbon-12 23 −1 • NA = 6:02214129(27) × 10 mol How was Avogadro's Constant Invented? Avogadro's constant was invented because scientists were learning about measuring matter and wanted a way to relate the microscopic to the macroscopic (e.g. how many particles (atoms, molecules, etc.) were in a sample of matter). The first scientists to see a need for Avogadro's constant were studying gases and how they behave under different temperatures and pressures. Amedeo Avogadro did not invent the constant, but he did state that all gases with the same volume, pressure, and temperature contained the same number of gas particles, which turned out to be very important to the development of our understanding of the principles of chemistry and physics. The constant was first calculated by Johann Josef Loschmidt, a German scientist, in 1865. He actually calculated the Loschmidt number, a constant that measures the same thing as Avogadro's number, but in different units (ideal gas particles per cubic meter at 0◦C and 1 atm). When converted to the same units, his number was off by about a factor of 10 from Avogadro's number. That may sound like a lot, but given the tools he had to work with for both theory and experiment and the magnitude of the number (∼ 1023), that is an impressively close estimate. -

2.1 Relative Atomic and Molecular Masses, the Avogadro Constant, and the Mole Calculations AQA Chemistry
2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations The Avogadro constant Specification reference 3.1.2.2 MS 0.1 Recognise and use expressions in decimal and ordinary form MS 0.4 Use calculators to find and use power, exponential, and logarithmic functions MS 1.1Use an appropriate number of significant figures. Learning objectives After completing the worksheet you should be able to: carry out calculations using the Avogadro constant carry out calculations using numbers in standard and ordinary form substitute numerical values into algebraic equations, and change the subject of equations report calculations to an appropriate number of significant figures. Introduction One mole of any substance contains the same number of particles as the number of atoms in 12 g of carbon-12. This number of particles is a huge number and is called the Avogadro constant. It has a value of 6.02 1023 and is given the units of mol−1 (per mole). The relative atomic mass of an element in grams contains 6.02 1023 atoms or one mole of that element. The relative formula mass of a compound in grams contains 6.02 1023 particles (molecules or ions) or one mole of that compound. Therefore we use the term molar mass to describe the relative atomic or formula mass of a substance in grams and give it the unit g mol−1. To calculate the number of particles in a certain mass of a substance we can use the following equation. mass m (g) Number of particles = ´ 6.02 ´ 1023(mol-1) molar mass M (g mol-1) Worked example Question 1 How many molecules are there in 42.5 g of ammonia, NH3? Answer © Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements This resource sheet may have been changed from the original 1 2.1 Relative atomic and molecular AQA Chemistry masses, the Avogadro constant, and the mole Calculations Step 1 Calculate the molar mass of ammonia using the relative atomic masses of nitrogen and hydrogen from the periodic table. -

The Mole and Avogadro's Number
The Mole and Avogadro's Number The name mole (German Mol) is attributed to Wilhelm Ostwald who introduced the concept in the year 1902. It is an abbreviation for molecule (German Molekül), which is in turn derived from Latin moles "mass, massive structure". (From the Wikipedia article on the mole unit.) A good site that introduces the mole concept and includes sample calculations and practice problems can be found here, from John Park's excellent ChemTeam site. For some interesting background on Avogadro's number, see here. By T.A. Furtsch, Tennessee Technological University, Cookeville, TN. Don't miss the interview with Count Amedeo Avogadro and his wife the Countess Felicita, located here (thanks to Kory Tonouchi, Roosevelt High, Honolulu, HI). Avogadro's 1811 publication, "Essay on a Manner of Determining the Relative Masses of the Elementary Molecules of Bodies, and the Proportions in Which They Enter into These Compounds", may be found here (thanks to Carmen Giunta, Le Moyne College, Syracuse, NY). A fun "mole" page to visit is here. It is a collection of student projects from Carondelet High School. Back in '98 they had a mole mystery described here. Did you know we have a mole day? Find out about it here. And for some corny mole jokes, don't miss the Dictionary of Mole Day Terms & Jokes here! Introduction A mole of objects contains Avogadro's number, 6.022 X 1023, objects. Just as a dozen apples is 12 apples, a mole of apples is 6.022 X 1023 apples. A mole of iron atoms is 6.022 X 1023 iron atoms. -
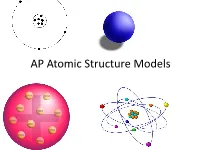
AP Atomic Structure Models What Is a Model?
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept. Models A model in science is usually an approximation of the “real thing.” This means that all models have simplifications that may lead to omitted information. Models When a model is selected for a specific purpose, it should be able to help predict a “behavior.” The Four Historical Models of the Atom 1. Particle model 2. Plum Pudding model 3. Rutherford model 4. Bohr model Modern Model Quantum Mechanical Model Why do we have so many historical models? Are some of them still useful? When we initially began the process of determining the structure of the atom, we first had to overcome the prevailing scientific concept that matter was continuous. This idea of continuous matter was from Aristotle. (a Greek philosopher 384-322 B.C.E.) Particle Model John Dalton’s four ideas about the particle nature of matter helped push forward the acceptance of the first model. 1. All elements are composed of tiny indivisible particles called atoms. 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. John Dalton 3. Atoms of different elements can physically mix together or can chemically combine in simple whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element, however, are never changed into atoms of another element as a result of a chemical reaction. -

Chemistry-I Content
CLASS-11 th THE CENTRAL BOARD OF SECONDARY EDUCATION CHEMISTRY-I CONTENT Unit 1 1 Some basic concepts of chemistry Unit 2 21 Structure of Atom Unit 3 51 Classification of element and Periodicity in Properties Unit 4 80 Chemical bonding and molecular structure Unit 5 111 States of matter: Gases and Liquids Unit 6 134 Chemical Thermodynamics Unit 7 164 Equilibrium CHAPTER 1‒SOME BASIC CONCEPTS OF CHEMISTRY Content V Nature of matter V Properties of matter and their measurement V Uncertainty in measurement V Laws of chemical combination V Dalton’s atomic theory V Atomic and molecular masses V Percentage composition V Stoichiometry and stoichiometric calculation V Important Questions Importance of Chemistry 1) Many types of drugs, fairness products, medicines and many more things which we use in daily life are because of chemistry. 2) Drugs effective in cancer therapy – Cisplatin and Taxol. 3) Drug used for AIDS patients-AZT (azidothymidine). Nature of Matter Matter It is defined as “Anything that has mass, occupies space and can be felt by any one or more senses is called matter”. Physical classification of matter On the basis of physical properties matter can be classified into five states as discussed below 1) Solid 2) Liquid 3) Gas 4) Plasma (highest energetic state of matter) 5) BEC (Bose - Einstein condensate) But we usually study only first three because of their vast existence in our environment. Properties of matter 1) Solid It possesses definite shape and definite volume which means it cannot be compressed on applying pressure on it. www.toppersnotes.com 1 • Fixed shape and volume • Intermolecular space is very low • Intermolecular forces are very strong • They have rigidity (resistance to change in shape) • Rocks, metal, wood, Iron, copper sulphate, bricks etc.