Embolization for Hemoptysis—Angiographic Anatomy of Bronchial and Systemic Arteries
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Variations of the Subclavian Artery and Its Branches Ahmet H
Okajimas Folia Anat. Jpn., 76(5): 255-262, December, 1999 The Variations of the Subclavian Artery and Its Branches By Ahmet H. YUCEL, Emine KIZILKANAT and CengizO. OZDEMIR Department of Anatomy, Faculty of Medicine, Cukurova University, 01330 Balcali, Adana Turkey -Received for Publication, June 19,1999- Key Words: Subclavian artery, Vertebral artery, Arterial variation Summary: This study reports important variations in branches of the subclavian artery in a singular cadaver. The origin of the left vertebral artery was from the aortic arch. On the right side, no thyrocervical trunk was found. The two branches which normally originate from the thyrocervical trunk had a different origin. The transverse cervical artery arose directly from the subclavian artery and suprascapular artery originated from the internal thoracic artery. This variation provides a short route for posterior scapular anastomoses. An awareness of this rare variation is important because this area is used for diagnostic and surgical procedures. The subclavian artery, the main artery of the The variations of the subclavian artery and its upper extremity, also gives off the branches which branches have a great importance both in blood supply the neck region. The right subclavian arises vessels surgery and in angiographic investigations. from the brachiocephalic trunk, the left from the aortic arch. Because of this, the first part of the right and left subclavian arteries differs both in the Subjects origin and length. The branches of the subclavian artery are vertebral artery, internal thoracic artery, This work is based on a dissection carried out in thyrocervical trunk, costocervical trunk and dorsal the Department of Anatomy in the Faculty of scapular artery. -

The Descending Thoracic Aorta Morphological Characteristics
ARS Medica Tomitana - 2016; 3(22): 186 - 191 10.1515/arsm-2016-0031 Malik S., Bordei P., Rusali A., Iliescu D. M. The descending thoracic aorta morphological characteristics Faculty of Medicine, “Ovidius” University, Constanta ABSTRACT Introduction Our study was conducted by consulting angioCT sites made on a CT GE LightSpeed VCT64 Slice CT and a CT GE LightSpeed 16 Slice CT, following the path and relationships of the descending thoracic aorta against the vertebral column, outside diameters thereof at the Descending thoracic aorta extends from the thoracic vertebrae T4, T7, T12 and posterior intercostal aortic arch (which it continues) and aortic hiatus of arteries characteristics. The origin of of the descending the diaphragm at level of T12 vertebra [1,2,3,4,5] thoracic aorta we found most commonly on the left corresponding to the front of T10 [6], level that flank of the lower edge of the vertebral body T4, but continues with the abdominal descending aorta. She I have encountered cases where it had come above the enters the posterior mediastinum at the T4 vertebra and lower edge of T4 on level of intervertebral disc T4-T5 or describes a trajectory which is vertically downward as even at the upper edge of T5 vertebral body. At thoracic a whole, being slightly inferior oblique and to the right, vertebra T4, on a total of 30 cases, the descending thoracic aorta present a diameter of 20.0 to 32.6 mm, then, first at a distance of 2-3 cm midline, progressive values that correspond to male gender and to females approach to become median and prevertebral at the diameter ranging from 25.5 to 27, 4 mm. -

Intercostal Arteries a Single Posterior & Two Anterior Intercostal Arteries
Intercostal Arteries •Each intercostal space contains: . A single posterior & .Two anterior intercostal arteries •Each artery gives off branches to the muscles, skin, parietal pleura Posterior Intercostal Arteries In the upper two spaces, arise from the superior intercostal artery (a branch of costocervical trunk of the subclavian artery) In the lower nine spaces, arise from the branches of thoracic aorta The course and branching of the intercostal arteries follow the intercostal Posterior intercostal artery Course of intercostal vessels in the posterior thoracic wall Anterior Intercostal Arteries In the upper six spaces, arise from the internal thoracic artery In the lower three spaces arise from the musculophrenic artery (one of the terminal branch of internal thoracic) Form anastomosis with the posterior intercostal arteries Intercostal Veins Accompany intercostal arteries and nerves Each space has posterior & anterior intercostal veins Eleven posterior intercostal and one subcostal vein Lie deepest in the costal grooves Contain valves which direct the blood posteriorly Posterior Intercostal Veins On right side: • The first space drains into the right brachiocephalic vein • Rest of the intercostal spaces drain into the azygos vein On left side: • The upper three spaces drain into the left brachiocephalic vein. • Rest of the intercostal spaces drain into the hemiazygos and accessory hemiazygos veins, which drain into the azygos vein Anterior Intercostal Veins • The lower five spaces drain into the musculophrenic vein (one of the tributary of internal thoracic vein) • The upper six spaces drain into the internal thoracic vein • The internal thoracic vein drains into the subclavian vein. Lymphatics • Anteriorly drain into anterior intercostal nodes that lie along the internal thoracic artery • Posterioly drain into posterior intercostal nodes that lie in the posterior mediastinum . -

Aorta and the Vasculature of the Thorax
Aorta and the Vasculature of the Thorax Ali Fırat Esmer, MD Ankara University Faculty of Medicine Department of Anatomy THE AORTA After originating from left ventricle, it ascends for a short distance, arches backward and to the left side, descends within the thorax on the left side of the vertebral column It is divided for purposes of The aorta is the main arterial trunk description into: that delivers oxygenated blood from Ascending aorta the left ventricle of the heart to the Arch of the aorta and tissues of the body. Descending aorta (thoracic and abdominal aorta) Ascending Aorta The ascending aorta begins at the base of the left ventricle runs upward and forward at the level of the sternal angle, where it becomes continuous with the arch of the aorta it possesses three bulges, the sinuses of the aorta Branches Right coronary artery Left coronary artery ARCH OF THE AORTA The aortic arch is a continuation of the ascending aorta and begins at the level of the second sternocostal joint. • It arches superiorly, posteriorly and to the left before moving inferiorly. • The aortic arch ends at the level of the T4 vertebra / at level of sternal angle. Branches; Brachiocephalic artery (Innominate artery) Left common carotid artery Left subclavian artery It begins when the ascending aorta emerges from the pericardial sac and courses upward, backward, and to the left as it passes through the superior mediastinum, ending on the left side at vertebral level TIV/V. Extending as high as the midlevel of the manubrium of the sternum, the arch is initially anterior and finally lateral to the trachea. -

Ascending and Descending Thoracic Vertebral Arteries
CLINICAL REPORT EXTRACRANIAL VASCULAR Ascending and Descending Thoracic Vertebral Arteries X P. Gailloud, X L. Gregg, X M.S. Pearl, and X D. San Millan ABSTRACT SUMMARY: Thoracic vertebral arteries are anastomotic chains similar to cervical vertebral arteries but found at the thoracic level. Descending thoracic vertebral arteries originate from the pretransverse segment of the cervical vertebral artery and curve caudally to pass into the last transverse foramen or the first costotransverse space. Ascending thoracic vertebral arteries originate from the aorta, pass through at least 1 costotransverse space, and continue cranially as the cervical vertebral artery. This report describes the angiographic anatomy and clinical significance of 9 cases of descending and 2 cases of ascending thoracic vertebral arteries. Being located within the upper costotransverse spaces, ascending and descending thoracic vertebral arteries can have important implications during spine inter- ventional or surgical procedures. Because they frequently provide radiculomedullary or bronchial branches, they can also be involved in spinal cord ischemia, supply vascular malformations, or be an elusive source of hemoptysis. ABBREVIATIONS: ISA ϭ intersegmental artery; SIA ϭ supreme intercostal artery; VA ϭ vertebral artery he cervical portion of the vertebral artery (VA) is formed by a bral arteria lusoria8-13 or persistent left seventh cervical ISA of Tseries of anastomoses established between the first 6 cervical aortic origin.14 intersegmental arteries (ISAs) and one of the carotid-vertebral This report discusses 9 angiographic observations of descend- anastomoses, the proatlantal artery.1-3 The VA is labeled a “post- ing thoracic VAs and 2 cases of ascending thoracic VAs. costal” anastomotic chain (ie, located behind the costal process of cervical vertebrae or dorsal to the rib itself at the thoracic level) to CASE SERIES emphasize its location within the transverse foramina. -

Product Information
G30 Latin VASA CAPITIS et CERVICIS ORGANA INTERNA 1 V. frontalis 49 Pulmo sinister 2 V. temporalis superficialis 50 Atrium dextrum 3 A. temporalis superficialis 51 Atrium sinistrum 3 a A. maxillaris 52 Ventriculus dexter 4 A. occipitalis 53 Ventriculus sinister 5 A. supratrochlearis 54 Valva aortae 6 A. et V. angularis 55 Valva trunci pulmonalis 7 A. et V. facialis 56 Septum interventriculare 7 a A. lingualis 57 Diaphragma 9 V. retromandibularis 58 Hepar 10 V. jugularis interna 11 A. thyroidea superior VASA ORGANORUM INTERNORUM 12 A. vertebralis 59 Vv. hepaticae 13 Truncus thyrocervicalis 60 V. gastrica dextra et sinistra 14 Truncus costocervicalis 61 A. hepatica communis 15 A. suprascapularis 61 a Truncus coeliacus 16 A. et V. subclavia dextra 62 V. mesenterica superior 17 V. cava superior 63 V. cava inferior 18 A. carotis communis 64 A. et V. renalis 18 a A. carotis externa 65 A. mesenterica superior 19 Arcus aortae 66 A. et V. lienalis 20 Pars descendens aortae 67 A. gastrica sinistra 68 Pars abdominalis® aortae VASA MEMBRII SUPERIORIS 69 A. mesenterica inferior 21 A. et V. axillaris 22 V. cephalica VASA REGIONIS PELVINAE 22 a A. circumflexa humeri anterior 72 A. et V. iliaca communis 22 b A. circumflexa humeri posterior 73 A. et V. iliaca externa 23 A. thoracodorsalis 74 A. sacralis mediana 24 A. et V. brachialis 75 A. et V. iliaca interna 25 A. thoracoacromialis 26 A. subclavia sinistra VASA MEMBRI INFERIORIS 27 V. basilica 76 Ramus ascendens a. circumflexae femoris 28 A. collateralis ulnaris superior lateralis 29 A. ulnaris 77 Ramus descendens a. -

An Unusual Origin and Course of the Thyroidea Ima Artery, with Absence of Inferior Thyroid Artery Bilaterally
Surgical and Radiologic Anatomy (2019) 41:235–237 https://doi.org/10.1007/s00276-018-2122-1 ANATOMIC VARIATIONS An unusual origin and course of the thyroidea ima artery, with absence of inferior thyroid artery bilaterally Doris George Yohannan1 · Rajeev Rajan1 · Akhil Bhuvanendran Chandran1 · Renuka Krishnapillai1 Received: 31 May 2018 / Accepted: 21 October 2018 / Published online: 25 October 2018 © Springer-Verlag France SAS, part of Springer Nature 2018 Abstract We report an unusual origin and course of the thyroidea ima artery in a male cadaver. The ima artery originated from the right subclavian artery very close to origin of the right vertebral artery. The artery coursed anteriorly between the common carotid artery medially and internal jugular vein laterally. It then coursed obliquely, from below upwards, from lateral to medial superficial to common carotid artery, to reach the inferior pole of the right lobe of thyroid and branched repeatedly to supply the anteroinferior and posteroinferior aspects of both the thyroid lobes and isthmus. The superior thyroid arteries were normal. Both the inferior thyroid arteries were absent. The unusual feature of this thyroidea ima artery is its origin from the subclavian artery close to vertebral artery origin, the location being remarkably far-off from the usual near midline position, and the oblique and relatively superficial course. This report is a caveat to neck surgeons to consider such a superficially running vessel to be a thyroidea ima artery. Keywords Thyroid vascular anatomy · Thyroidea ima artery · Artery of Neubauer · Blood supply of thyroid · Variations Introduction (1.1%), transverse scapular (1.1%), or pericardiophrenic or thyrocervical trunk [8, 10]. -
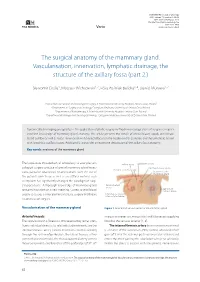
The Surgical Anatomy of the Mammary Gland. Vascularisation, Innervation, Lymphatic Drainage, the Structure of the Axillary Fossa (Part 2.)
NOWOTWORY Journal of Oncology 2021, volume 71, number 1, 62–69 DOI: 10.5603/NJO.2021.0011 © Polskie Towarzystwo Onkologiczne ISSN 0029–540X Varia www.nowotwory.edu.pl The surgical anatomy of the mammary gland. Vascularisation, innervation, lymphatic drainage, the structure of the axillary fossa (part 2.) Sławomir Cieśla1, Mateusz Wichtowski1, 2, Róża Poźniak-Balicka3, 4, Dawid Murawa1, 2 1Department of General and Oncological Surgery, K. Marcinkowski University Hospital, Zielona Gora, Poland 2Department of Surgery and Oncology, Collegium Medicum, University of Zielona Gora, Poland 3Department of Radiotherapy, K. Marcinkowski University Hospital, Zielona Gora, Poland 4Department of Urology and Oncological Urology, Collegium Medicum, University of Zielona Gora, Poland Dynamically developing oncoplasty, i.e. the application of plastic surgery methods in oncological breast surgeries, requires excellent knowledge of mammary gland anatomy. This article presents the details of arterial blood supply and venous blood outflow as well as breast innervation with a special focus on the nipple-areolar complex, and the lymphatic system with lymphatic outflow routes. Additionally, it provides an extensive description of the axillary fossa anatomy. Key words: anatomy of the mammary gland The large-scale introduction of oncoplasty to everyday on- axillary artery subclavian artery cological surgery practice of partial mammary gland resec- internal thoracic artery thoracic-acromial artery tions, partial or total breast reconstructions with the use of branches to the mammary gland the patient’s own tissue as well as an artificial material such as implants has significantly changed the paradigm of surgi- cal procedures. A thorough knowledge of mammary gland lateral thoracic artery superficial anatomy has taken on a new meaning. -

Anatomy of Esophagus Anatomy of Esophagus
DOI: 10.5772/intechopen.69583 Provisional chapter Chapter 1 Anatomy of Esophagus Anatomy of Esophagus Murat Ferhat Ferhatoglu and Taner Kıvılcım Murat Ferhat Ferhatoglu and Taner Kıvılcım Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/intechopen.69583 Abstract Anatomy knowledge is the basic stone of healing diseases. Arteries, veins, wall structure, nerves, narrowing, curves, relations with other organs are very important to understand eso- phagial diseases. In this chapter we aimed to explain anatomical fundementals of oesophagus. Keywords: anatomy, esophagus, parts of esophagus, blood supply of esophagus, innervation of esophagus 1. Introduction Esophagus is a muscular tube-like organ that originates from endodermal primitive gut, 25–28 cm long, approximately 2 cm in diameter, located between lower border of laryngeal part of pharynx (Figure 1) and cardia of stomach. Start and end points of esophagus correspond to 6th cervi- cal vertebra and 11th thoracic vertebra topographically, and the gastroesophageal junction cor- responds to xiphoid process of sternum. Five cm of esophagus is in the neck, and it descends over superior mediastinum and posterior mediastinum approximately 17–18 cm, continues for 1–1.5 cm in diaphragm, ending with 2–3 cm of esophagus in abdomen (Figure 2) [1, 2]. Sex, age, physi- cal condition, and gender affect the length of esophagus. A newborn’s esophagus is 18 cm long, and it begins and ends one or two vertebra higher than in adult. Esophagus lengthens to 22 cm long by age 3 years and to 27 cm by age 10 years [3, 4]. -

An Aberrant Right Lateral Branch from Right Internal Thoracic Artery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Directory of Open Access Journals eISSN 1308-4038 International Journal of Anatomical Variations (2010) 3: 114–116 Case Report An aberrant right lateral branch from right internal thoracic artery Published online August 10th, 2010 © http://www.ijav.org Vishal Manoharrao SALVE ABSTRACT Challa RATNAPRABHA The internal thoracic artery is the largest artery of the thoracic wall. The internal thoracic artery is often mobilized for coronary artery bypass grafting. During routine dissection (MBBS Batch 2009-2010) of a middle aged male cadaver at Dr. Pinnamaneni Siddhartha Institute of Medical Sciences & Research Foundation, Gannavaram, (INDIA); an aberrant right lateral branch from right internal thoracic artery was found. It arose Department of Anatomy, Dr. Pinnamaneni Siddhartha Institute of Medical Sciences from right internal thoracic artery behind right first rib. It ran downward for first four intercostal spaces & Research Foundation, Chinnaoutpalli, Gannavaram Mandal, Krishna District (AP), about 1 cm away from the mid-axillary line. It terminated into two intercostal arteries on either side in the INDIA. 4th intercostal space. The rare and unexpected occurrence of variation of the internal thoracic artery such as the one reported here may complicate the entire procedure of coronary artery by-pass grafting. Thus this rare variant of the internal thoracic artery is of great concern during any surgical procedure that involves this artery. © IJAV. 2010; 3: 114–116. Vishal Manoharrao Salve, MBBS, MS Assistant Professor Department of Anatomy Dr. Pinnamaneni Siddhartha Institute of Medical Sciences & Research Foundation Chinnaoutpalli, Gannavaram Mandal Krishna District (AP), 521286, INDIA. -

Variations of the Subclavian Arterial Branching Pattern and Maximization of Its Juwan Ryu Western University, [email protected]
Western University Scholarship@Western Masters of Clinical Anatomy Projects Anatomy and Cell Biology Department 2016 Variations of the Subclavian Arterial Branching Pattern and Maximization of its Juwan Ryu Western University, [email protected] Follow this and additional works at: https://ir.lib.uwo.ca/mcap Part of the Anatomy Commons Citation of this paper: Ryu, Juwan, "Variations of the Subclavian Arterial Branching Pattern and Maximization of its" (2016). Masters of Clinical Anatomy Projects. 14. https://ir.lib.uwo.ca/mcap/14 Variations of the Subclavian Arterial Branching Pattern and Maximization of its Supraclavicular Surgical Exposure (Project format: Integrated) by Juwan Ryu Graduate Program in Anatomy and Cell Biology Division of Clinical Anatomy A project submitted in partial fulfillment of the requirements for the degree of Master’s of Science The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada © Juwan Ryu 2016 Abstract The subclavian artery (SCA) is an important vessel with several branches. However, significant pattern variations exist. Characterizing SCA branches and its relationships to landmark structures like the anterior scalene muscle (ASM) is important in surgery. Computed Tomography Angiograms from 55 patients were retrospectively analyzed using Aquarius iNtuition. Measurements were taken of: distance of origin of SCA branches from the aorta and the ASM-VA origin distance. Only 13 SCAs (12.9%) exhibited the highest prevalence in typical branching pattern. VA originated 1st in 80.2% of SCAs, with ITA arising 2nd (41.3%), TCT 3rd (47.3%), CCT 4th (43.6%) and DSA 5th branch (56.9%). Average VA-ASM distance was 14.14mm with 94.9% of VAs originating within 30mm proximal to the medial border of ASM. -

The Dorsal Scapular Artery - a Proposed Term for the Artery to the Rhomboid Muscles
The Dorsal Scapular Artery - A Proposed Term for the Artery to the Rhomboid Muscles DONALD F. HUELKE Department of Anatomy, The University of Michigan, Medical School, Ann Arbor, Michigan The terminology of the arterial supply from the thyrocervical trunk in 77.5% of to the rhomboid and trapezius muscles is sides). (2) That the artery which sup- quite confusing in that each artery has plies the rhomboid muscles take the name been given two different names; names of the nerve along with which it passes, which are related to the variations in the and be designated the dorsal scapular ar- origin of these vessels. According to the tery. This artery arises from the second recent Paris Revision of the terminology or third part of the subclavian artery, sepa- (’55) one of these is the transverse cer- rate from the transverse cervical artery, vical artery, of the thyrocervical trunk, in approximately 70% of the sides. Thus, which gives rise to a superficial and a only one term is used for each artery. deep branch to the trapezius and rhom- If this modification in terminology is to boid muscles respectively. When these be accepted internationally, there must be branches arise separately, the trapezial an agreement among anatomists as to its branch is called the “superficial cervical “usual” site of origin. Only by comparing artery” and that to the rhomboids, the data on the origins of these arteries from “descending scapular artery.” Thus, each various countries can this end be achieved. vessel has two different names. Generally, Therefore, it is the purpose of this report an artery is named by the area of supply, to compare the data on these vessels of irrespective of its origin or variations.