The Salmonids of Bristol Bay 41
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bristol Bay, Alaska
EPA 910-R-14-001C | January 2014 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska Volume 3 – Appendices E-J Region 10, Seattle, WA www.epa.gov/bristolbay EPA 910-R-14-001C January 2014 AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J U.S. Environmental Protection Agency Region 10 Seattle, WA CONTENTS VOLUME 1 An Assessment of Potential Mining Impacts on Salmon Ecosystems of Bristol Bay, Alaska VOLUME 2 APPENDIX A: Fishery Resources of the Bristol Bay Region APPENDIX B: Non-Salmon Freshwater Fishes of the Nushagak and Kvichak River Drainages APPENDIX C: Wildlife Resources of the Nushagak and Kvichak River Watersheds, Alaska APPENDIX D: Traditional Ecological Knowledge and Characterization of the Indigenous Cultures of the Nushagak and Kvichak Watersheds, Alaska VOLUME 3 APPENDIX E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values APPENDIX F: Biological Characterization: Bristol Bay Marine Estuarine Processes, Fish, and Marine Mammal Assemblages APPENDIX G: Foreseeable Environmental Impact of Potential Road and Pipeline Development on Water Quality and Freshwater Fishery Resources of Bristol Bay, Alaska APPENDIX H: Geologic and Environmental Characteristics of Porphyry Copper Deposits with Emphasis on Potential Future Development in the Bristol Bay Watershed, Alaska APPENDIX I: Conventional Water Quality Mitigation Practices for Mine Design, Construction, Operation, and Closure APPENDIX J: Compensatory Mitigation and Large-Scale Hardrock Mining in the Bristol Bay Watershed AN ASSESSMENT OF POTENTIAL MINING IMPACTS ON SALMON ECOSYSTEMS OF BRISTOL BAY, ALASKA VOLUME 3—APPENDICES E-J Appendix E: Bristol Bay Wild Salmon Ecosystem: Baseline Levels of Economic Activity and Values Bristol Bay Wild Salmon Ecosystem Baseline Levels of Economic Activity and Values John Duffield Chris Neher David Patterson Bioeconomics, Inc. -
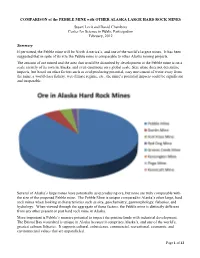
COMPARISON of the PEBBLE MINE with OTHER ALASKA LARGE HARD ROCK MINES
COMPARISON of the PEBBLE MINE with OTHER ALASKA LARGE HARD ROCK MINES Stuart Levit and David Chambers Center for Science in Public Participation February, 2012 Summary If permitted, the Pebble mine will be North America’s, and one of the world’s largest mines. It has been suggested that in spite of its size the Pebble mine is comparable to other Alaska mining projects. The amount of ore mined and the area that would be disturbed by development at the Pebble mine is on a scale entirely of its own in Alaska, and even enormous on a global scale. Size alone does not determine impacts, but based on other factors such as acid producing potential, easy movement of water away from the mine, a world class fishery, wet climate regime, etc., the mine’s potential impacts could be significant and irreparable. Several of Alaska’s large mines have potentially acid producing ore, but none are truly comparable with the size of the proposed Pebble mine. The Pebble Mine is unique compared to Alaska’s other large, hard rock mines when looking at characteristics such as size, geochemistry, geomorphology, fisheries, and hydrology. When viewed through the aggregate of these factors, the Pebble mine is distinctly different from any other present or past hard rock mine in Alaska. More important is Pebble’s massive potential to impact the pristine lands with industrial development. The Bristol Bay watershed is unique in Alaska because it comprises Alaska’s, and one of the world’s, greatest salmon fisheries. It supports cultural, subsistence, commercial, recreational, economic, and environmental values that are unparalleled. -

Comment on G Marty Dcoument
Critique of the Document “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions” Presented to the Provincial Government of British Columbia by Gary Marty, D.V.M., Ph.D., Diplomate, A.C.V.P. of the British Columbia Ministry of Agriculture, Animal Health Centre, Abbotsford. Authors of this critique: Lawrence M. Dill1, Martin Krkosek2, Brendan Connors3, Stephanie J. Peacock4, Andrew W. Bateman5, Richard Routledge6, Mark A. Lewis7, and John Reynolds8 1 Professor Emeritus, Department of Biological Sciences, Simon Fraser University 2 Assistant Professor, Department of Ecology and Evolutionary Biology, University of Toronto 3 Senior Systems Ecologist, ESSA Technologies, and Adjunct Professor, Department of Biological Sciences, Simon Fraser University 4 PhD Candidate, Department of Biological Sciences, University of Alberta 5 Postdoctoral Fellow, Department of Biological Sciences, University of Alberta and Department of Ecology and Evolutionary Biology, University of Toronto 6 Professor, Department of Statistics and Actuarial Science, Simon Fraser University 7 Professor and Senior Canada Research Chair, Departments of Biological Sciences and Mathematical and Statistical Sciences, University of Alberta 8 Professor and Tom Buell BC Leadership Chair in Aquatic Conservation, Department of Biological Sciences, Simon Fraser University Background The document, “Information Regarding Concerns about Farmed Salmon - Wild Salmon Interactions,” dated March 16, 2015, was presented to Ministers Thompson and Letnik of the Government of British Columbia (BC) with the intention of providing scientific information upon which to base management and policy decisions regarding wild and farmed salmon in British Columbia. Collectively, we are a group of scientists, mostly academic, whose research expertise includes salmon and infectious diseases (here we refer to infectious diseases in the broadest sense as those that may arise from parasitic, viral or bacterial pathogens). -
Lake Huron Spawning
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Petition to List the Iliamna Lake Seal, a Distinct Population Segment of Eastern North Pacific Harbor Seal (Phoca Vitulina Richardii), Under the U.S
Before the Secretary of Commerce Petition to List the Iliamna Lake Seal, a Distinct Population Segment of Eastern North Pacific Harbor Seal (Phoca vitulina richardii), under the U.S. Endangered Species Act Photo Credit: NOAA Fisheries/Dave Withrow Center for Biological Diversity 6 February 2020 i Notice of Petition Wilbur Ross, Secretary of Commerce U.S. Department of Commerce 1401 Constitution Ave. NW Washington, D.C. 20230 Email: [email protected], [email protected] Dr. Neil Jacobs, Acting Under Secretary of Commerce for Oceans and Atmosphere U.S. Department of Commerce 1401 Constitution Ave. NW Washington, D.C. 20230 Email: [email protected] Petitioner: Kristin Carden, Oceans Program Scientist, on behalf of the Center for Biological Diversity 1212 Broadway #800 Oakland, CA 94612 Phone: 510.844.7100 x327 Email: [email protected] On November 19, 2012, the Center for Biological Diversity (Center, Petitioner) submitted to the Secretary of Commerce and the National Oceanographic and Atmospheric Administration (NOAA) through the National Marine Fisheries Service (NMFS) a petition to list the Iliamna Lake population of eastern North Pacific harbor seal (Phoca vitulina richardii) as threatened or endangered under the U.S. Endangered Species Act (ESA). (See generally Center 2012.) On May 17, 2013, NMFS issued a positive 90- day finding “that the petition present[ed] substantial scientific or commercial information indicating that the petition action may be warranted” and initiated a status review. (78 Fed. Reg. 29,098 (May 17, 2013).). On November 17, 2016, NMFS issued a determination that listing was not warranted because “the seals in Iliamna Lake do not constitute a species, subspecies, or distinct population segment (DPS) under the ESA.” (81 Fed. -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

Walrus Hunting at Togiak, Bristol Bay, Soutwest Alaska
WALRUS HUNTING AT TOGIAK, BRISTOL BAY, SOUTHWEST ALASKA James A. Fall, Molly Chythlook, Janet Schichnes, and Rick Sinnott’ Technical Paper No. 212 ’ Fall: Division of Subsistence, Anchorage Chythlook and Schichnes: Division of Subsistence, Dillingham Sinnott: Division of Wildlife Conservation, Anchorage Alaska Department of Fish and Game Division of Subsistence Juneau, Alaska October 1991 The Alaska Department of Fish and Game operates all of its public programs and activities free from discrimination on the basis of race, religion, color, national origin, age, sex, or handicap. Because the department receives federal funding, any person who believes he or she has been discriminated against should write to: O.E.D. U.S. Department of the Interior Washington, D.C. 20240 , ABSTRACT The report provides an overview of historic and contemporary uses of Pacific walrus in the Bristol Bay region of southwest Alaska. It focuses on the community of Togiak (population 613) and its traditional use areas, including Round Island (Yup’ik Qayaciq, “place to go in a kayak”). As part of the Walrus Islands State Game Sanctuary, Round Island, a critical haul out site, has been closed to walrus hunting since 1960. In 1991, the Togiak Traditional Council submitted a proposal to the Alaska Board of Game to allow a limited hunt for 10 walrus on Round Island in October. Hunting of walrus and other marine mammals in western Bristol Bay, including Round Island, by the Native people of the Togiak area over the last 2,500 years is documented by archaeological and ethnohistorical evidence. Until the late 1930s and early 1940% well-organized groups of hunters from Togiak traveled in kayaks to Round Island (the most reliable hunting location) and other islands armed with spears and harpoons to harvest walrus. -

ORPC Rivgen Wake Characterization
ORPC RivGen Wake Characterization Maricarmen Guerra Paris* Jim Thomson University of Washington University of Washington Seattle, WA, USA Seattle, WA, USA *Corresponding author: [email protected] in turbulence due to eddies shed by turbine blades; and iii) complex interactions between natural and turbine ABSTRACT induced turbulent structures [6]. In this investigation we assess the wake formed behind Baseline and post-deployment flow conditions were mea- a horizontal cross-flow turbine installed on the Kvichak sured at the ORPC RivGen turbine site on the Kvichak river in southwest Alaska, USA, just downstream of the river in the vicinity of Igiugig village, Alaska. Mean sur- village of Igiugig. The small village is home to 70 peo- face flow and turbulence measurements were collected ple and its electricity source currently depends on an from a drifting platform equipped with a Nortek Signa- isolated power grid fed by diesel generators. The Ocean ture 1000Hz five beam AD2CP. Baseline measurements Renewable Power Company (ORPC) has set a pilot hy- indicate a maximum flow of 2.5 m/s and a 10% turbu- drokinetic energy project on the Kvichak river stream to lent intensity in the turbine vicinity. Measurements af- provide Igiugig with a renewable and locally produced ter turbine deployment and grid connection show a sig- source of energy. nificant decrease in surface velocity up to 200 m down- ORPCs RivGen turbine was successfully deployed, stream from the turbine and an increase in turbulence tested and connected to the local power grid during intensity up to 20% that extends about 75 m down- the summers of 2014 and 2015. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Summary Report No
Canadian Manuscript Report of Fisheries and Aquatic Sciences 2614 2002 Life History Characteristics Of Freshwater Fishes Occurring in the Northwest Territories and Nunavut, With Major Emphasis on Riverine Habitat Requirements by C.L. Evans1, J.D. Reist1 and C.K. Minns2 1. Department of Fisheries and Oceans, Arctic Fish Ecology and Assessment Research, Central and Arctic Division, 501 University Crescent, Winnipeg, Manitoba, R3T 2N6 Canada 2. Department of Fisheries and Oceans, Great Lakes Laboratory of Fisheries and Aquatic Sciences, Bayfield Institute, 867 Lakeshore Road, P.O. Box 5050, Burlington, Ontario, L7R 4A6 Canada. Her Majesty the Queen in Right of Canada, 2002 Cat. No. Fs 97-4/2614E ISSN 0706-6473 Correct citation of this publication: Evans, C.E., J.D. Reist and C.K. Minns. 2002. Life history characteristics of freshwater fishes occurring in the Northwest Territories and Nunavut, with major emphasis on riverine habitat requirements. Can. MS Rep. Fish. Aquat. Sci. 2614: xiii + 169 p. ii TABLE OF CONTENTS LIST OF FIGURES .......................................................................................................... v LIST OF TABLES............................................................................................................ v ABSTRACT ...................................................................................................................viii RÉSUMÉ ........................................................................................................................viii INTRODUCTION............................................................................................................ -

2014 Draft Fisheries Monitoring Plan
2014 Draft Fisheries Monitoring Plan TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 INTRODUCTION ..................................................................................................................... 2 Continuation Projects in 2014 ................................................................................................. 7 Technical Review Committee Membership .............................................................................. 8 Technical Review Committee, Regional Advisory Council, and Interagency Staff Committee Recommendations .................................................................................................................. 9 Summary of Regional Advisory Council Recommendations and Rationale .............................. 15 NORTHERN REGION OVERVIEW .................................................................................... 19 14-101 - Unalakleet River Chinook Salmon Assessment Continuation .................................... 25 14-102 - Climate change and subsistence fisheries: quantifying the direct effects of climatic warming on arctic fishes and lake ecosystems using whole-lake manipulations on the Alaska North Slope ........................................................................................................................... 27 14-103 - Dispersal patterns and summer ocean distribution of adult Dolly Varden in the Beaufort Sea using satellite telemetry .................................................................................. -

Spawning Distribution of Bering Ciscoes in the Yukon River
Transactions of the American Fisheries Society 144:292–299, 2015 American Fisheries Society 2015 ISSN: 0002-8487 print / 1548-8659 online DOI: 10.1080/00028487.2014.988881 ARTICLE Spawning Distribution of Bering Ciscoes in the Yukon River Randy J. Brown* and David W. Daum1 U.S. Fish and Wildlife Service, 101 12th Avenue, Room 110, Fairbanks, Alaska 99701, USA Abstract Bering Ciscoes Coregonus laurettae are anadromous salmonids with known spawning populations only in the Yukon, Kuskokwim, and Susitna rivers in Alaska. A commercial fishery for the species was recently initiated at the mouth of the Yukon River, inspiring a series of research projects to enhance our understanding of the exploited population. This study was designed to delineate the geographic spawning distribution of Bering Ciscoes in the Yukon River. One hundred radio transmitters per year in 2012 and 2013 were deployed in prespawning Bering Ciscoes at a site located 1,176 km upstream from the sea. A total of 160 fish survived fish wheel capture and tagging, avoided harvest and predation after tagging, and continued migrating upstream to their spawning destinations. Approximately 79% migrated to spawn in the upper Yukon Flats, upstream from the mouth of the Porcupine River, and 21% migrated to spawn in the lower Yukon Flats. Locating the Bering Cisco spawning area, which is almost entirely encompassed by the Yukon Flats National Wildlife Refuge, enhances our ability to protect it from anthropogenic disturbance and enables future biological research on the spawning population. Conservation of migratory fish in large rivers requires an removing gravel from fish spawning habitats has been shown understanding of habitat use across a species’ range and the to reduce spawning success (Fudge and Bodaly 1984; Meng ability to manage anthropogenic impacts to essential habitats and Muller€ 1988), which could jeopardize the viability of such as migration routes and spawning areas (Gross 1987; affected populations.