Thesis-1993D-R359d.Pdf (822.8Kb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Arizona Department of Agriculture Environmental & Plant Services Division 1688 W
DOUGLAS A. DUCEY MARK W. KILLIAN Governor Director Arizona Department of Agriculture Environmental & Plant Services Division 1688 W. Adams Street, Phoenix, Arizona 85007 P. (602) 542-0994 F. (602) 542-1004 SUMMARY OF EXTERIOR QUARANTINES Updated April 16, 2021 CONTACTS Jack Peterson…...…………………………………………………………………………..…Associate Director (602) 542-3575 [email protected] Rachel Paul…………………………………………………………………………...Field Operations Manager (602) 542-3243 [email protected] Jamie Legg………………………………………………………………………..Quarantine Program Manager (602) 542-0992 [email protected] INDEX Summaries………………………………………………………………………………………...……….Page 2 Nursery Stock…………………………………………………………………………...…………Page 2 House Plants……………………………………………………………………………………….Page 2 Boll Weevil Pest…………………………………………………………………………………...Page 2 Citrus Nursery Stock Pests………………………………………………………………………...Page 3 Nut Tree Pests……………………………………………………………………………………..Page 3 Nut Pests…………………………………………………………………………………………...Page 4 Lettuce Mosaic Virus……………………………………………………………………………...Page 4 Imported Fire Ants………………………………………………………………………………...Page 5 Palm Tree Pests…………………………………………………………………………………....Page 5 Noxious Weeds…………………………………………………………………………………....Page 7 Japanese beetle…………………………………………………………………………………….Page 9 Arizona Administrative Code, Title 3, Chapter 4, Article 2 Quarantine……………………..………….Page 10 April 16, 2021 www.agriculture.az.gov Page 1 SUMMARIES Nursery Stock States Regulated - All states, districts, and territories of the United States. Regulated Commodities - All trees, shrubs, vines, cacti, agaves, succulents, -

Hickory Shuckworm
INSECT PESTS Hickory Shuckworm Prepared by Katherine McIntyre, MG 2007 Camille Goodwin, MG 2008 Texas AgriLife Extension Service Galveston County Office Dickinson, TX 77539 Educational programs of the Texas AgriLife Extension Service are open to all people without regard to race, color, sex, disability, religion, age, or national origin. The Texas A&M System, U.S. Department of Agriculture and the County Commissioners Courts of Texas cooperating. FIG. 1 Type Pest: chewing insect (Cydia caryana Fitch) Type Metamorphous: complete (egg, larva, pupa, adult stages) Period of Primary Occurrence: early June until harvest • Adults are most active at night and because of size are difficult to find • Larvae feed on young developing nut Plants Affected • Pecan and hickory FIG. 2 Identifying Characteristics of Insect Pest LARVAE / EGG STAGE • Larvae stage white with brown heads about ½" long (Fig. 1) • Larvae make tunnels in the shuck (before shell hardens), interrupting flow of nutrients and water necessary for normal kernel development (Fig. 2 & 3) ADULT STAGE • Adult stage small, gray to smoky-black colored moth • Dimensions 3/8" long with wing span of ½" wide Description / Symptoms FIG. 3 • Primary insect pest of pecans in the Galveston Country area and the most economically damaging pecan insect pest in southwest Texas • Premature nut drop…before shell hardens (Fig. 7 & 8) • Poor kernel development, shuck sticking, scarring and discoloring of the shell and delayed nut maturity occur from shuck mining activity of larvae after shell hardening (Fig. 4) • Except for premature nut drop, early stages of shuckworm damage goes unnoticed unless the shuck is cut open to reveal larvae tunneling • Shuckworms do not impact the health of pecan trees (just the quality of the nut crop) FIG. -

U.S. EPA, Pesticide Product Label, SPLAT CYDIA, 05/24/2007
go~r6 - 3 • Cydia For Mating Disruption of the Codling Moth, Cydia pomonella and Hickory Shuckworm, Cydia caryana SPLA T (Specialized Pheromone and Lure Application Technology) is an amorphous polymer matrix (or the sustained release of insect pheromones. SPLA T Cydia provides control by disrupting mating behavior. ACTIVE INGREDIENT: Codling Moth Technical Pheromone (E,E)-8, 10-Dodecadlen-1-o1; .......................... 10.0% OTHER INGREDIENTS: ....................................... 90.0% Total: .................................................................. 100.0% KEEP OUT OF REACH OF CHILDREN CAUTION See side/back panel for Additional Precautionary Statements EPA Reg. No. 80286- Net Contents: Ibs _kg EPA Est No. 80286-CA-003 FIRST AID If in eyes: • Hold eye open and rinse slowly and gently with water for 15-20 minutes. • Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. • Call poison control center or doctor for treatment advice. If on skin or clothing: • Take off contaminated clothing. • Wash skin immediately with soap and water and rinse with plenty of water for 5-10 minutes. • Call poison control center or doctor for treatment advice. If swallowed: • Call poison control center or doctor immediately for treatment advice. • Halle person sip a glass of water if able to swallow. • Do not induce vomiting unless advised to do so by poison control center or doctor. • Do not give anything by mouth to an unconscious person. Hotline Number Have the product container or label with you when calling a poison control center or doctor, or going for treatment. FOR MEDICAL EMERGENCY INFORMATION CALL 1-800-222- 1222. '-CCE'PTED ----- MAY 242007 -- .--. PRECAUTIONARY STATEMENTS Hazard to Humans and Domestic Animals: CAUTION. -

Arizona Administrative Code Between the Dates of October 1, 2020 Through December 31, 2020 (Supp
3 A.A.C. 4 Supp. 20-4 December 31, 2020 Title 3 TITLE 3. AGRICULTURE CHAPTER 4. DEPARTMENT OF AGRICULTURE - PLANT SERVICES DIVISION The table of contents on the first page contains quick links to the referenced page numbers in this Chapter. Refer to the notes at the end of a Section to learn about the history of a rule as it was published in the Arizona Administrative Register. Sections, Parts, Exhibits, Tables or Appendices codified in this supplement. The list provided contains quick links to the updated rules. This Chapter contains rule Sections that were filed to be codified in the Arizona Administrative Code between the dates of October 1, 2020 through December 31, 2020 (Supp. 20-4). Table 1. Fee Schedule ......................................................47 Questions about these rules? Contact: Name: Brian McGrew Address: Department of Agriculture 1688 W. Adams St. Phoenix, AZ 85007 Telephone: (602) 542-3228 Fax: (602) 542-1004 E-mail: [email protected] Website: https://agriculture.az.gov/plantsproduce/industrial- hemp-program The release of this Chapter in Supp. 20-4 replaces Supp. 20-3, 1-50 pages Please note that the Chapter you are about to replace may have rules still in effect after the publication date of this supplement. Therefore, all superseded material should be retained in a separate binder and archived for future reference. i PREFACE Under Arizona law, the Department of State, Office of the Secretary of State (Office), accepts state agency rule filings and is the publisher of Arizona rules. The Office of the Secretary of State does not interpret or enforce rules in the Administrative Code. -

Seeds for Sowing:MNSV Measures Draft Ihs
Seeds for Sowing 155.02.05 15 February 2021 Import Health Standard Issued under the Biosecurity Act 1993 Import Health Standard: Seeds for Sowing 15/02/2021 TITLE Import Health Standard: Seeds for Sowing COMMENCEMENT This consolidated standard comes into force on [date]. This import health standard amends the Import Health Standard: Seeds for Sowing, which came into force on 15 February 2021, and consolidates all amendments made up to commencement of this notice. The amendment history to this import health standard is set out in [Appendix 2] ISSUING AUTHORITY This Import Health Standard is issued under section 24A of the Biosecurity Act 1993 and incorporates amendments made in accordance with section 24B(1)(a) of that Act. Dated at Wellington this day of 2021 Director, Animal & Plant Health Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Animal & Plant Health Directorate Plant Imports PO Box 2526 Wellington 6140 Email: [email protected] Ministry for Primary Industries Page 1 of 171 Import Health Standard: Seeds for Sowing 15/02/2021 Contents Page Introduction 5 Part 1: General Requirements 7 1.1 Application 7 1.2 Incorporation of material by reference 7 1.3 Definitions 7 1.4 Requirements for seed for sowing 7 1.5 Documentation 9 1.6 Post - entry quarantine 11 1.7 Seed for sowing of New Zealand origin 12 1.8 Seed for sowing imported as laboratory specimens 12 1.9 Seed imported as pelleted seed 13 Part 2: Specific Requirements -

1 Modern Threats to the Lepidoptera Fauna in The
MODERN THREATS TO THE LEPIDOPTERA FAUNA IN THE FLORIDA ECOSYSTEM By THOMSON PARIS A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2011 1 2011 Thomson Paris 2 To my mother and father who helped foster my love for butterflies 3 ACKNOWLEDGMENTS First, I thank my family who have provided advice, support, and encouragement throughout this project. I especially thank my sister and brother for helping to feed and label larvae throughout the summer. Second, I thank Hillary Burgess and Fairchild Tropical Gardens, Dr. Jonathan Crane and the University of Florida Tropical Research and Education center Homestead, FL, Elizabeth Golden and Bill Baggs Cape Florida State Park, Leroy Rogers and South Florida Water Management, Marshall and Keith at Mack’s Fish Camp, Susan Casey and Casey’s Corner Nursery, and Michael and EWM Realtors Inc. for giving me access to collect larvae on their land and for their advice and assistance. Third, I thank Ryan Fessendon and Lary Reeves for helping to locate sites to collect larvae and for assisting me to collect larvae. I thank Dr. Marc Minno, Dr. Roxanne Connely, Dr. Charles Covell, Dr. Jaret Daniels for sharing their knowledge, advice, and ideas concerning this project. Fourth, I thank my committee, which included Drs. Thomas Emmel and James Nation, who provided guidance and encouragement throughout my project. Finally, I am grateful to the Chair of my committee and my major advisor, Dr. Andrei Sourakov, for his invaluable counsel, and for serving as a model of excellence of what it means to be a scientist. -

Predator to Prey to Poop: Bats As Microbial Hosts and Insectivorous Hunters
Predator to Prey to Poop: Bats as Microbial Hosts and Insectivorous Hunters A Thesis SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA BY Miranda Galey IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE Dr. Ron Moen, Dr. Jessica R. Sieber September 2020 Copyright © Miranda Galey 2020 Abstract Bat fecal samples are a rich source of ecological data for bat biologists, entomologists, and microbiologists. Feces collected from individual bats can be used to profile the gut microbiome using microbial DNA and to understand bat foraging strategies using arthropod DNA. We used eDNA collected from bat fecal samples to better understand bats as predators in the context of their unique gut physiology. We used high through- put sequencing of the COI gene and 16S rRNA gene to determine the diet composition and gut microbiome composition of three bat species in Minnesota: Eptesicus fuscus, Myotis lucifugus and M. septentrionalis. In our analysis of insect prey, we found that E. fuscus consistently foraged for a higher diversity of beetle species compared to other insects. We found that the proportional frequency of tympanate samples from M. septentrionalis and M. lucifugus was similar, while M. septentrionalis consistently preyed more often upon non-flying species. We used the same set of COI sequences to determine presence of pest species, rare species, and insects not previously observed in Minnesota. We were able to combine precise arthropod identification and the for- aging areas of individually sampled bats to observe possible range expansion of some insects. The taxonomic composition of the bat gut microbiome in all three species was found to be consistent with the composition of a mammalian small intestine. -
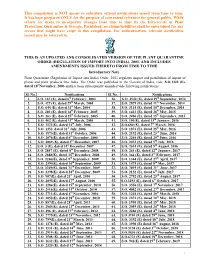
This Compilation Is NOT Meant to Substitute Official Notifications Issued from Time to Time
This compilation is NOT meant to substitute official notifications issued from time to time. It has been prepared ONLY for the purpose of convenient reference for general public. While efforts are made to incorporate changes from time to time by the Directorate of Plant Protection, Quarantine & Storage, Faridabad, no claims/liabilities shall be entertained for any errors that might have crept in this compilation. For authentication, relevant notification issued may be referred to. THIS IS AN UPDATED AND CONSOLIDATED VERSION OF THE PLANT QUARANTINE ORDER (REGULATION OF IMPORT INTO INDIA), 2003, AND INCLUDES AMENDMENTS ISSUED THERETO FROM TIME TO TIME Introductory Note Plant Quarantine (Regulation of Import into India) Order, 2003 regulates import and prohibition of import of plants and plant products into India. The Order was published in the Gazette of India, vide, S.O.1322 (E), dated 18thNovember, 2003 and has been subsequently amended vide following notifications: Sl. No. Notifications Sl. No. Notifications 1. S.O. 167 (E), dated 6th February, 2004 36. S.O. 2542 (E), dated 29th September, 2014 2. S.O. 427 (E), dated 29th March, 2004 37. S.O. 2879 (E), dated 11th November, 2014 3. S.O. 644 (E), dated 31st May, 2004 38. S.O. 3114 (E), dated 10th December, 2014 4. S.O. 203 (E), dated 14th February, 2005 39. S.O. 1413 (E), dated 26th May, 2015 5. S.O. 263 (E), dated 25th February, 2005 40. S.O. 2496 (E), dated 15th September, 2015 6. S.O. 462 (E), dated 31st March, 2005 41. S.O. 101(E), dated 13th January, 2016 7. -

Seed for Sowing
MAF BIOSECURITY NEW ZEALAND STANDARD 155.02.05 Importation of Seed for Sowing Issued as an import health standard pursuant to section 22 of the Biosecurity Act 1993 MAF Biosecurity New Zealand Ministry of Agriculture and Forestry P O Box 2526 Wellington New Zealand CONTENTS Review and Endorsement Amendment Record 1 Introduction 1.1 Scope 1.2 References 1.3 Definitions And Abbreviations 1.4 General 1.5 Appendices 1.5.1 Quarantine Impurities 1.5.2 Schedule Of Regulated (Quarantine) Weed Seeds 2 Import Specification And Entry Conditions 2.1 Import Specification 2.1.1 For Quarantine Pests Other Than Weed Seeds: 2.1.2 For Quarantine Weed Seeds: 2.2 Entry Conditions 2.2.1 Categories Of Entry Conditions 2.2.2 Basic Conditions 2.2.2.1 Cleanliness 2.2.2.2 Labelling 2.2.2.3 Phytosanitary Certificate 2.2.2.4 Seed Analysis 2.2.3 Importation Of Pelleted Seed 2.2.3.1 Additional Declarations 2.2.3.2 Seed Analysis 2.2.4 Importation Of Seed In Hermetically Sealed Containers/Packages 2.2.5 Importation Of Seed Mixtures 3. Schedule Of Species Requiring Additional Declarations And/Or Post Entry Quarantine 3.1 Notes To The Schedule 3.2 Permit To Import 3.3 Importation Of Seed Into Post Entry Quarantine 3.4 Amendments To The Plants Biosecurity Index 3.5 Schedule Of Special Conditions MAF Biosecurity New Zealand Standard 155.02.05: Importation of Seed for 3 December 2007 Page 2 Sowing REVIEW This MAF Biosecurity New Zealand standard is subject to ongoing review. -

Butterflies of North America
Insects of Western North America 4. Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. Part 3 Chapter 1 Survey of Spiders (Arachnida, Araneae) of Fort Sill, Comanche Co., Oklahoma Chapter 2 Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. III. Arachnida: Ixodidae, Scorpiones, Hexapoda: Ephemeroptera, Hemiptera, Homoptera, Coleoptera, Neuroptera, Trichoptera, Lepidoptera, and Diptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University 1 Cover Photo Credits: The Black and Yellow Argiope, Argiope aurantia Lucas, (Photo by P.E. Cushing), a robber fly Efferia texana (Banks) (Photo by C. Riley Nelson). ISBN 1084-8819 Information about the availability of this publication and others in the series may be obtained from Managing Editor, C.P. Gillette Museum of Arthropod Ddiversity, Department of Bbioagricultural Sciences and Pest Management, Colorado State University, Ft. Collins, CO 80523-1177 2 Insects of Western North America 4. Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. III Edited by Paul A. Opler Chapter 1 Survey of Spiders (Arachnida, Araneae) of Fort Sill, Comanche Co., Oklahoma by Paula E. Cushing and Maren Francis Department of Zoology, Denver Museum of Nature and Science Denver, Colorado 80205 Chapter 2 Survey of Selected Arthropod Taxa of Fort Sill, Comanche County, Oklahoma. III. Arachnida: Ixodidae, Scorpiones, Hexapoda: Ephemeroptera, Hemiptera, Homoptera, Coleoptera, Neuroptera, Trichoptera, Lepidoptera, and Diptera by Boris C. Kondratieff, Jason P. Schmidt, Paul A. Opler, and Matthew C. Garhart C.P. Gillette Museum of Arthropod Diversity Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, Colorado 80523 January 2005 Contributions of the C.P. -

To Download the Food Plant Database in PDF Format
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Abelia spathulata Siebold & Zucc. Caprifoliaceae Acleris askoldana (Christoph) Tortricinae Yasuda 1972 Asia Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982; Nasu & Yasuda 1993 Asia Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Crocidosema plebejana Zeller Olethreutinae Heinrich 1921; Diakonoff 1982 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota nigrocervina Walsingham Tortricinae MacKay 1962a North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Platynota rostrana (Walker) Tortricinae Heinrich 1921 North America Moench (as Hibiscus) Abelmoschus esculentus (L.) Malvaceae Archips micaceana (Walker) Tortricinae Pholboon 1965; Kuroko & Lewvanich 1993 Asia Moench Abelmoschus esculentus (L.) Malvaceae Archips philippa (Meyrick) Tortricinae BMNH collection Asia India Moench Abelmoschus esculentus (L.) Malvaceae Homona tabescens (Meyrick) Tortricinae Yunus & Ho 1980 Asia Malaysia Moench Abelmoschus esculentus Moench Malvaceae Crocidosema plebejana Zeller Olethreutinae Fletcher 1932 Asia India (as Hibiscus) Abelmoschus esculentus Moench Thaumatotibia leucotreta (Meyrick) (as Malvaceae Olethreutinae Whittle 1984 Africa (as Hibiscus) Cryptophlebia) Abies alba Mill. Pinaceae Acleris variana (Fernald) Tortricinae Meyrick MS 1938 North America Abies alba Mill. Pinaceae Archips oporana (Linnaeus) Tortricinae Bradley et al. 1973 Europe Abies