Nphp1 Invs (Nphp2) Nphp3 Nphp4 Nphp5 (Iqcb1) Nphp6 (Cep290) Tmem67 (Nphp11)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educational Paper Ciliopathies
Eur J Pediatr (2012) 171:1285–1300 DOI 10.1007/s00431-011-1553-z REVIEW Educational paper Ciliopathies Carsten Bergmann Received: 11 June 2011 /Accepted: 3 August 2011 /Published online: 7 September 2011 # The Author(s) 2011. This article is published with open access at Springerlink.com Abstract Cilia are antenna-like organelles found on the (NPHP) . Ivemark syndrome . Meckel syndrome (MKS) . surface of most cells. They transduce molecular signals Joubert syndrome (JBTS) . Bardet–Biedl syndrome (BBS) . and facilitate interactions between cells and their Alstrom syndrome . Short-rib polydactyly syndromes . environment. Ciliary dysfunction has been shown to Jeune syndrome (ATD) . Ellis-van Crefeld syndrome (EVC) . underlie a broad range of overlapping, clinically and Sensenbrenner syndrome . Primary ciliary dyskinesia genetically heterogeneous phenotypes, collectively (Kartagener syndrome) . von Hippel-Lindau (VHL) . termed ciliopathies. Literally, all organs can be affected. Tuberous sclerosis (TSC) . Oligogenic inheritance . Modifier. Frequent cilia-related manifestations are (poly)cystic Mutational load kidney disease, retinal degeneration, situs inversus, cardiac defects, polydactyly, other skeletal abnormalities, and defects of the central and peripheral nervous Introduction system, occurring either isolated or as part of syn- dromes. Characterization of ciliopathies and the decisive Defective cellular organelles such as mitochondria, perox- role of primary cilia in signal transduction and cell isomes, and lysosomes are well-known -
Shimada 2017.Pdf
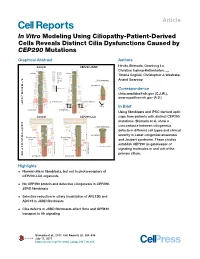
Article In Vitro Modeling Using Ciliopathy-Patient-Derived Cells Reveals Distinct Cilia Dysfunctions Caused by CEP290 Mutations Graphical Abstract Authors Control CEP290-JSRD Hiroko Shimada, Quanlong Lu, Christine Insinna-Kettenhofen, ..., Cilium GPR161 Cilium ADCY3 Tiziana Cogliati, Christopher J. Westlake, Smo ARL13B F CEP290 Cell membrane Anand Swaroop I Gli2 B Cytoplasm R Other proteins O Correspondence Axoneme B Axoneme [email protected] (C.J.W.), L A [email protected] (A.S.) S Cell membrane Cell membrane T Vesicles In Brief Cytoplasm Cytoplasm Using fibroblasts and iPSC-derived optic Control CEP290-LCA cups from patients with distinct CEP290 mutations, Shimada et al. show a P H Cell membrane concordance between ciliogenesis O T ? defects in different cell types and clinical Axoneme ? O ? Axoneme ? Cytoplasm severity in Leber congenital amaurosis R ? Connecting Connecting ? E Cilium Cilium ? and Joubert syndrome. These studies Cell membrane C Cell membrane E establish CEP290 as gatekeeper of P Docked mother centriole T ?? ? ? signaling molecules in and out of the O primary cilium. R Cytoplasm Cytoplasm Highlights d Normal cilia in fibroblasts, but not in photoreceptors of CEP290-LCA organoids d No CEP290 protein and defective ciliogenesis in CEP290- JSRD fibroblasts d Selective reduction in ciliary localization of ARL13B and ADCY3 in JSRD fibroblasts d Cilia defects in JSRD fibroblasts affect Smo and GPR161 transport in Hh signaling Shimada et al., 2017, Cell Reports 20, 384–396 July 11, 2017 http://dx.doi.org/10.1016/j.celrep.2017.06.045 Cell Reports Article In Vitro Modeling Using Ciliopathy-Patient-Derived Cells Reveals Distinct Cilia Dysfunctions Caused by CEP290 Mutations Hiroko Shimada,1 Quanlong Lu,2 Christine Insinna-Kettenhofen,2 Kunio Nagashima,3 Milton A. -

The Emerging Landscape of Dynamic DNA Methylation in Early Childhood
The emerging landscape of dynamic DNA methylation in early childhood Cheng-Jian Xu, Marc Jan Bonder, Cilla Söderhäll, Mariona Bustamante, Nour Baïz, Ulrike Gehring, Soesma Jankipersadsing, Pieter van der Vlies, Cleo van Diemen, Bianca van Rijkom, et al. To cite this version: Cheng-Jian Xu, Marc Jan Bonder, Cilla Söderhäll, Mariona Bustamante, Nour Baïz, et al.. The emerg- ing landscape of dynamic DNA methylation in early childhood. BMC Genomics, BioMed Central, 2017, 18, pp.25. 10.1186/s12864-016-3452-1. hal-01792686 HAL Id: hal-01792686 https://hal.archives-ouvertes.fr/hal-01792686 Submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Xu et al. BMC Genomics (2017) 18:25 DOI 10.1186/s12864-016-3452-1 RESEARCHARTICLE Open Access The emerging landscape of dynamic DNA methylation in early childhood Cheng-Jian Xu1,2*, Marc Jan Bonder2, Cilla Söderhäll3,4, Mariona Bustamante5,6,7,8, Nour Baïz9, Ulrike Gehring10, Soesma A. Jankipersadsing1,2, Pieter van der Vlies2, Cleo C. van Diemen2, Bianca van Rijkom2, Jocelyne Just9,11, Inger Kull12, Juha Kere3,13, Josep Maria Antó5,7,8,14, Jean Bousquet15,16,17,18, Alexandra Zhernakova2, Cisca Wijmenga2, Isabella Annesi-Maesano9, Jordi Sunyer5,7,8,14, Erik Melén19, Yang Li2*, Dirkje S. -

Unraveling the Genetics of Joubert and Meckel-Gruber Syndromes
Journal of Pediatric Genetics 3 (2014) 65–78 65 DOI 10.3233/PGE-14090 IOS Press Unraveling the genetics of Joubert and Meckel-Gruber syndromes Katarzyna Szymanska, Verity L. Hartill and Colin A. Johnson∗ Department of Ophthalmology and Neuroscience, University of Leeds, Leeds, UK Received 27 May 2014 Revised 11 July 2014 Accepted 14 July 2014 Abstract. Joubert syndrome (JBTS) and Meckel-Gruber syndrome (MKS) are recessive neurodevelopmental conditions caused by mutations in proteins that are structural or functional components of the primary cilium. In this review, we provide an overview of their clinical diagnosis, management and molecular genetics. Both have variable phenotypes, extreme genetic heterogeneity, and display allelism both with each other and other ciliopathies. Recent advances in genetic technology have significantly improved diagnosis and clinical management of ciliopathy patients, with the delineation of some general genotype-phenotype correlations. We highlight those that are most relevant for clinical practice, including the correlation between TMEM67 mutations and the JBTS variant phenotype of COACH syndrome. The subcellular localization of the known MKS and JBTS proteins is now well-described, and we discuss some of the contemporary ideas about ciliopathy disease pathogenesis. Most JBTS and MKS proteins localize to a discrete ciliary compartment called the transition zone, and act as structural components of the so-called “ciliary gate” to regulate the ciliary trafficking of cargo proteins or lipids. Cargo proteins include enzymes and transmembrane proteins that mediate intracellular signaling. The disruption of transition zone function may contribute to the ciliopathy phenotype by altering the composition of the ciliary membrane or axoneme, with impacts on essential developmental signaling including the Wnt and Shh pathways as well as the regulation of secondary messengers such as inositol-1,4,5-trisphosphate (InsP3) and cyclic adenosine monophosphate (cAMP). -

Prenatal Exome Sequencing in Anomalous Fetuses: New Opportunities and Challenges
© American College of Medical Genetics and Genomics ORIGINAL RESEARCH ARTICLE Prenatal exome sequencing in anomalous fetuses: new opportunities and challenges Neeta L. Vora, MD1, Bradford Powell, MD, PhD2, Alicia Brandt, MS2, Natasha Strande, PhD3,4, Emily Hardisty, MS, CGC1, Kelly Gilmore, MS, CGC1, Ann Katherine M. Foreman, MS, CGC2,5, Kirk Wilhelmsen, MD, PhD6, Chris Bizon, PhD6, Jason Reilly, BA6, Phil Owen, BS6, Cynthia M. Powell, MD, MS2,7, Debra Skinner, PhD, MA8, Christine Rini, PhD9, Anne D. Lyerly, MD, MA10, Kim A. Boggess, MD1, Karen Weck, MD3,4, Jonathan S. Berg, MD, PhD2 and James P. Evans, MD, PhD2,10 Purpose: We investigated the diagnostic and clinical performance the following genes: COL1A1, MUSK, KCTD1, RTTN, TMEM67, of exome sequencing in fetuses with sonographic abnormalities PIEZO1 and DYNC2H1. One additional case revealed a de novo with normal karyotype and microarray and, in some cases, normal nonsense mutation in a novel candidate gene (MAP4K4). gene-specific sequencing. The perceived likelihood that exome sequencing would explain Methods: Exome sequencing was performed on DNA from 15 the results (5.2 on a 10-point scale) was higher than the anomalous fetuses and from the peripheral blood of their approximately 30% diagnostic yield discussed in pretest counseling. parents. Parents provided consent to be informed of diagnostic Conclusion: Exome sequencing had diagnostic utility in a highly results in the fetus, medically actionable findings in the parents, select population of fetuses where a genetic diagnosis was highly and their identification as carrier couples for significant auto- suspected. Challenges related to genetics literacy and variant somal recessive conditions. -

GENETIC STUDY of RENAL DISEASES (Nephroref Global®) by MASSIVE SEQUENCING (NGS)
Pablo Iglesias, 57 – Polígono Gran Via Sur 08908 L'Hospitalet de Llobregat (Barcelona) Tel. 932 593 700 – Fax. 932 845 000 GENETIC STUDY OF RENAL DISEASES (NephroRef Global®) BY MASSIVE SEQUENCING (NGS) Request No.: 000 Client: - Analysis code: 55580 Patient Name: xxx Date of Birth: N/A Patient Ref.: xxx Gender: Female Sample Type: Blood EDTA Sample Arrival Date: DD/MM/AAAA Date of Result: DD/MM/AAAA Clinical information: A 9-year-old patient with a nephrotic syndrome without response to corticosteroid therapy. She has nephrotic-range proteinuria with microhematuria, hypoalbuminemia with hypercholesterolemia and normal glomerular filtration. Paternal aunt with cortico-resistant nephrotic syndrome with evolution to end-stage renal failure that required renal transplantation at age 13. RESULT AND INTERPRETATION The presence of a heterozygous likely pathogenic variant has been identified. In addition, the presence of a heterozygous variant of uncertain clinical significance (VUS) has been identified.(See Interpretation and recommendations) The complete list of studied genes is available in Annex 1. (Methodology) The list of reported genes and coverage details is available in Table 1. (Methodology) Gene Variant* Zygosity Inheritance pattern Classification^ NPHS2 NM_014625.3: c.842A>C Heterozygosis Autosomal Recessive Likely Patogénica p.(Glu281Ala) INF2 NM_022489.3: c.67T>A Heterozygosis Autosomal Recessive VUS p.(Ser23Thr) * Nomenclature according to HGVS v15.11 ^ Based on the recommendations of the American College of Medical Genetics and Genomics (ACMG) Physician, technical specialist responsible for Clinical Analysis: Jaime Torrents Pont. The results relate to samples received and analysed. This report may not be reproduced in part without permission. This document is addressed to the addressee and contains confidential information. -

Leber Congenital Amaurosis Due to CEP290 Mutations – Severe Vision Impairment with a High Unmet Medical Need: a Review Author and Journal Details: Bart P
This plain-language summary (or PLS) describes a paper on Leber congenital amaurosis 10 that was published in a medical journal called “Retina”. Title of the paper: Leber congenital amaurosis due to CEP290 mutations – severe vision impairment with a high unmet medical need: a review Author and journal details: Bart P. Leroy, David G. Birch, Jacque L. Duncan, Byron L. Lam, Robert K. Koenekoop, Fernanda B. O. Porto, Stephen R. Russel, Aniz Girach. Retina 2021;doi:10.1097/IAE.0000000000003133. Online ahead of print. What does this paper report on? • The authors of this paper reviewed what scientists know about the genetic eye disease called Leber congenital amaurosis 10 (also known as LCA10). They looked at the scientific articles on this subject that have been published in high-quality medical journals Why was this research needed? • Tying together separate pieces of scientific evidence about a disease can help us to understand the disease better. It also helps identify where there may be areas of care for people with the disease that need to be improved. We call these gaps “unmet medical needs” • Bringing evidence together in this way is especially important for rare diseases as it improves awareness among healthcare professionals of the needs of people living with the disease The authors of the paper are expert eye doctors working at specialist centers in Belgium, Brazil, Canada, and the USA; one of the authors is an employee of ProQR Therapeutics. This PLS has been developed in collaboration with Bart Leroy (an author on the original paper), Francesca Diodati and Matthew Carr, with medical writing assistance provided by ApotheCom. -

Ciliopathies Gene Panel
Ciliopathies Gene Panel Contact details Introduction Regional Genetics Service The ciliopathies are a heterogeneous group of conditions with considerable phenotypic overlap. Levels 4-6, Barclay House These inherited diseases are caused by defects in cilia; hair-like projections present on most 37 Queen Square cells, with roles in key human developmental processes via their motility and signalling functions. Ciliopathies are often lethal and multiple organ systems are affected. Ciliopathies are London, WC1N 3BH united in being genetically heterogeneous conditions and the different subtypes can share T +44 (0) 20 7762 6888 many clinical features, predominantly cystic kidney disease, but also retinal, respiratory, F +44 (0) 20 7813 8578 skeletal, hepatic and neurological defects in addition to metabolic defects, laterality defects and polydactyly. Their clinical variability can make ciliopathies hard to recognise, reflecting the ubiquity of cilia. Gene panels currently offer the best solution to tackling analysis of genetically Samples required heterogeneous conditions such as the ciliopathies. Ciliopathies affect approximately 1:2,000 5ml venous blood in plastic EDTA births. bottles (>1ml from neonates) Ciliopathies are generally inherited in an autosomal recessive manner, with some autosomal Prenatal testing must be arranged dominant and X-linked exceptions. in advance, through a Clinical Genetics department if possible. Referrals Amniotic fluid or CV samples Patients presenting with a ciliopathy; due to the phenotypic variability this could be a diverse set should be sent to Cytogenetics for of features. For guidance contact the laboratory or Dr Hannah Mitchison dissecting and culturing, with ([email protected]) / Prof Phil Beales ([email protected]) instructions to forward the sample to the Regional Molecular Genetics Referrals will be accepted from clinical geneticists and consultants in nephrology, metabolic, laboratory for analysis respiratory and retinal diseases. -

Evidence of Oligogenic Inheritance in Nephronophthisis
JASN Express. Published on September 12, 2007 as doi: 10.1681/ASN.2007020243 CLINICAL RESEARCH www.jasn.org Evidence of Oligogenic Inheritance in Nephronophthisis Julia Hoefele,*† Matthias T.F. Wolf,* John F. O’Toole,* Edgar A. Otto,* Ulla Schultheiss,* Georges Deˆschenes,‡ Massimo Attanasio,* Boris Utsch,* Corinne Antignac,§ and ʈ Friedhelm Hildebrandt* ʈ Departments of *Pediatrics and Human Genetics, University of Michigan, Ann Arbor, Michigan; †Department of Pediatrics, University Children’s Hospital, University of Munich, Munich, Germany; and ‡Hoˆpital Robert Debre´, Pediatric Nephrology, AP-HP, and §INSERM, U574, Universite´ Paris Descartes, Faculte de Me´dicine Rene´ Descartes, and Hoˆpital Necker-Enfants Malades, AP-HP, Department of Genetics, Paris, France ABSTRACT Nephronophthisis is a recessive cystic renal disease that leads to end-stage renal failure in the first two decades of life. Twenty-five percent of nephronophthisis cases are caused by large homozygous deletions of NPHP1, but six genes responsible for nephronophthisis have been identified. Because oligogenic inheritance has been described for the related Bardet-Biedl syndrome, we evaluated whether mutations in more than one gene may also be detected in cases of nephronophthisis. Because the nephrocystins 1 to 4 are known to interact, we examined patients with nephronophthisis from 94 different families and sequenced all exons of the NPHP1, NPHP2, NPHP3, and NPHP4 genes. In our previous studies involving 44 families, we detected two mutations in one of the NPHP1–4 genes. Here, we detected in six families two mutations in either NPHP1, NPHP3, or NPHP4, and identified a third mutation in one of the other NPHP genes. Furthermore, we found possible digenic disease by detecting one individual who carried one mutation in NPHP2 and a second mutation in NPHP3. -

Renal Cystic Disorders Infosheet 6-14-19
Next Generation Sequencing Panel for Renal Cystic Disorders Clinical Features: Renal cystic diseases are a genetically heterogeneous group of conditions characterized By isolated renal disease or renal cysts in conjunction with extrarenal features (1). Age of onset of renal cystic disease ranges from neonatal to adult onset. Common features of renal cystic diseases include renal insufficiency and progression to end stage renal disease (ESRD). Identification of the genetic etiology of renal cystic disease can aid in appropriate clinical management of the affected patient. Our Renal Cystic Disorders Panel includes sequence and deletion/duplicaton analysis of all 79 genes listed below. Renal Cystic Disorders Sequencing Panel AHI1 BMPER HNF1B NEK8 TCTN3 WDPCP ANKS6 C5orf42 IFT27 NOTCH2 TFAP2A WDR19 ARL13B CC2D2A IFT140 NPHP1 TMEM107 XPNPEP3 ARL6 CDC73 IFT172 NPHP3 TMEM138 ZNF423 B9D1 CEP104 INPP5E NPHP4 TMEM216 B9D2 CEP120 INVS OFD1 TMEM231 BBIP1 CEP164 IQCB1 PDE6D TMEM237 BBS1 CEP290 JAG1 PKD2 TMEM67 BBS10 CEP41 KIAA0556 PKHD1 TRIM32 BBS12 CEP83 KIAA0586 REN TSC1 BBS2 CRB2 KIF14 RPGRIP1L TSC2 BBS4 CSPP1 KIF7 SALL1 TTC21B BBS5 DCDC2 LZTFL1 SDCCAG8 TTC8 BBS7 GLIS2 MKKS TCTN1 UMOD BBS9 GLIS3 MKS1 TCTN2 VHL Disorder Genes Inheritance Clinical features/molecular genetics Bardet Biedl ARL6 AR Bardet-Biedl syndrome (BBS) is an autosomal syndrome BBS1 recessive multi-systemic ciliopathy characterized By BBS10 retinal dystrophy, oBesity, postaxial polydactyly, BBS12 leaning difficulties, renal involvement and BBS2 genitourinary abnormalities (2). Visual prognosis is BBS4 poor, and the mean age of legal Blindness is 15.5 BBS5 years. Birth weight is typically normal But significant BBS7 weight gain Begins within the first year. Renal BBS9 disease is a major cause of morBidity and mortality. -

Ciliary Dyneins and Dynein Related Ciliopathies
cells Review Ciliary Dyneins and Dynein Related Ciliopathies Dinu Antony 1,2,3, Han G. Brunner 2,3 and Miriam Schmidts 1,2,3,* 1 Center for Pediatrics and Adolescent Medicine, University Hospital Freiburg, Freiburg University Faculty of Medicine, Mathildenstrasse 1, 79106 Freiburg, Germany; [email protected] 2 Genome Research Division, Human Genetics Department, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525 KL Nijmegen, The Netherlands; [email protected] 3 Radboud Institute for Molecular Life Sciences (RIMLS), Geert Grooteplein Zuid 10, 6525 KL Nijmegen, The Netherlands * Correspondence: [email protected]; Tel.: +49-761-44391; Fax: +49-761-44710 Abstract: Although ubiquitously present, the relevance of cilia for vertebrate development and health has long been underrated. However, the aberration or dysfunction of ciliary structures or components results in a large heterogeneous group of disorders in mammals, termed ciliopathies. The majority of human ciliopathy cases are caused by malfunction of the ciliary dynein motor activity, powering retrograde intraflagellar transport (enabled by the cytoplasmic dynein-2 complex) or axonemal movement (axonemal dynein complexes). Despite a partially shared evolutionary developmental path and shared ciliary localization, the cytoplasmic dynein-2 and axonemal dynein functions are markedly different: while cytoplasmic dynein-2 complex dysfunction results in an ultra-rare syndromal skeleto-renal phenotype with a high lethality, axonemal dynein dysfunction is associated with a motile cilia dysfunction disorder, primary ciliary dyskinesia (PCD) or Kartagener syndrome, causing recurrent airway infection, degenerative lung disease, laterality defects, and infertility. In this review, we provide an overview of ciliary dynein complex compositions, their functions, clinical disease hallmarks of ciliary dynein disorders, presumed underlying pathomechanisms, and novel Citation: Antony, D.; Brunner, H.G.; developments in the field. -

TULP1 Mutations Causing Early-Onset Retinal Degeneration: Preserved but Insensitive Macular Cones
Retina TULP1 Mutations Causing Early-Onset Retinal Degeneration: Preserved but Insensitive Macular Cones Samuel G. Jacobson,1 Artur V. Cideciyan,1 Wei Chieh Huang,1 Alexander Sumaroka,1 Alejandro J. Roman,1 Sharon B. Schwartz,1 Xunda Luo,1 Rebecca Sheplock,1 Joanna M. Dauber,1 Malgorzata Swider,1 and Edwin M. Stone2,3 1Scheie Eye Institute, Department of Ophthalmology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States 2Department of Ophthalmology, University of Iowa Carver College of Medicine, Iowa City, Iowa, United States 3Howard Hughes Medical Institute, Iowa City, Iowa, United States Correspondence: Samuel G. Jacob- PURPOSE. To investigate visual function and outer and inner retinal structure in the rare form of son, Scheie Eye Institute, University retinal degeneration (RD) caused by TULP1 (tubby-like protein 1) mutations. of Pennsylvania, 51 N. 39th Street, Philadelphia, PA 19104, USA; METHODS. Retinal degeneration patients with TULP1 mutations (n ¼ 5; age range, 5–36 years) [email protected]. were studied by kinetic and chromatic static perimetry, en face autofluorescence imaging, and spectral-domain optical coherence tomography (OCT) scans. Outer and inner retinal Submitted: April 10, 2014 Accepted: July 13, 2014 laminar thickness were measured and mapped across the central retina. Comparisons were made with results from patients with RD associated with four ciliopathy genotypes (MAK, Citation: Jacobson SG, Cideciyan AV, RPGR, BBS1, and USH2A). Huang WC, et al. TULP1 mutations causing early-onset retinal degenera- RESULTS. The TULP1-RD patients were severely affected already in the first decade of life and tion: preserved but insensitive macu- there was rapidly progressive visual loss.