Clinical Applications in Psychiatry Pharmacogenetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosulepin Prescribing
SAFETY BULLETIN: DOSULEPIN PRESCRIBING In December 2007, the Medicines and Healthcare Regulatory Agency (MHRA) issued safety advice around prescribing of dosulepin, related to the narrow margin between therapeutic doses and potentially fatal doses. Nevertheless, dosulepin continues to be prescribed widely. Over eight years after the safety advice was published, prescribing of dosulepin in the Dorset area remains high. In the South West area, Dorset CCG was the highest prescriber of dosulepin for the 2015/16 financial year (3.238% of all antidepressant items). Of the 209 CCGs in the UK, Dorset is the fourteenth highest prescriber of dosulepin, and well above the national average of 2.111%. The following points summarise the reasons that dosulepin is not recommended for prescribing nationally, and in Dorset: Dosulepin has a small margin of safety between the (maximum) therapeutic dose and potentially fatal doses. The NICE guideline on depression in adults recommends that dosulepin should not be prescribed for adults with depression because evidence supporting its tolerability relative to other antidepressants is outweighed by the increased cardiac risk and toxicity in overdose. Dosulepin has also been used ‘off label’ in other indications such as fibromyalgia and neuropathic pain. However the evidence for use in in this way is weak, and is not recommended. The lethal dose of dosulepin is relatively low and can be potentiated by alcohol and other CNS depressants. Dosulepin overdose is associated with high mortality and can occur rapidly, even before hospital treatment can be received. Onset of toxicity occurs within 4-6 hours. Every year, up to 200 people in England and Wales fatally overdose with dosulepin. -

Dose Equivalents of Antidepressants Evidence-Based Recommendations
Journal of Affective Disorders 180 (2015) 179–184 Contents lists available at ScienceDirect Journal of Affective Disorders journal homepage: www.elsevier.com/locate/jad Research report Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials Yu Hayasaka a,n, Marianna Purgato b, Laura R Magni c, Yusuke Ogawa a, Nozomi Takeshima a, Andrea Cipriani b,d, Corrado Barbui b, Stefan Leucht e, Toshi A Furukawa a a Department of Health Promotion and Human Behavior, Kyoto University Graduate School of Medicine/School of Public Health, Yoshida Konoe-cho, Sakyo- ku, Kyoto 606-8501, Japan b Department of Public Health and Community Medicine, Section of Psychiatry, University of Verona, Policlinico “G.B.Rossi”, Pzz.le L.A. Scuro, 10, Verona 37134, Italy c Psychiatric Unit, Istituto di Ricovero e Cura a Carattere Scientifico, Centro San Giovanni di Dio, Fatebenefratelli, Brescia, Italy d Department of Psychiatry, University of Oxford, Oxford, UK e Department of Psychiatry and Psychotherapy, Technische Universität München, Klinikum rechts der Isar, Ismaningerstr. 22, 81675 Munich, Germany article info abstract Article history: Background: Dose equivalence of antidepressants is critically important for clinical practice and for Received 4 February 2015 research. There are several methods to define and calculate dose equivalence but for antidepressants, Received in revised form only daily defined dose and consensus methods have been applied to date. The purpose of the present 10 March 2015 study is to examine dose equivalence of antidepressants by a less arbitrary and more systematic method. Accepted 12 March 2015 Methods: We used data from all randomized, double-blind, flexible-dose trials comparing fluoxetine or Available online 31 March 2015 paroxetine as standard drugs with any other active antidepressants as monotherapy in the acute phase Keywords: treatment of unipolar depression. -

Appendix A: Potentially Inappropriate Prescriptions (Pips) for Older People (Modified from ‘STOPP/START 2’ O’Mahony Et Al 2014)
Appendix A: Potentially Inappropriate Prescriptions (PIPs) for older people (modified from ‘STOPP/START 2’ O’Mahony et al 2014) Consider holding (or deprescribing - consult with patient): 1. Any drug prescribed without an evidence-based clinical indication 2. Any drug prescribed beyond the recommended duration, where well-defined 3. Any duplicate drug class (optimise monotherapy) Avoid hazardous combinations e.g.: 1. The Triple Whammy: NSAID + ACE/ARB + diuretic in all ≥ 65 year olds (NHS Scotland 2015) 2. Sick Day Rules drugs: Metformin or ACEi/ARB or a diuretic or NSAID in ≥ 65 year olds presenting with dehydration and/or acute kidney injury (AKI) (NHS Scotland 2015) 3. Anticholinergic Burden (ACB): Any additional medicine with anticholinergic properties when already on an Anticholinergic/antimuscarinic (listed overleaf) in > 65 year olds (risk of falls, increased anticholinergic toxicity: confusion, agitation, acute glaucoma, urinary retention, constipation). The following are known to contribute to the ACB: Amantadine Antidepressants, tricyclic: Amitriptyline, Clomipramine, Dosulepin, Doxepin, Imipramine, Nortriptyline, Trimipramine and SSRIs: Fluoxetine, Paroxetine Antihistamines, first generation (sedating): Clemastine, Chlorphenamine, Cyproheptadine, Diphenhydramine/-hydrinate, Hydroxyzine, Promethazine; also Cetirizine, Loratidine Antipsychotics: especially Clozapine, Fluphenazine, Haloperidol, Olanzepine, and phenothiazines e.g. Prochlorperazine, Trifluoperazine Baclofen Carbamazepine Disopyramide Loperamide Oxcarbazepine Pethidine -

Inhibitory Effect of Eslicarbazepine Acetate and S-Licarbazepine on 2 Nav1.5 Channels
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.24.059188; this version posted August 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Inhibitory effect of eslicarbazepine acetate and S-licarbazepine on 2 Nav1.5 channels 3 Theresa K. Leslie1, Lotte Brückner 1, Sangeeta Chawla1,2, William J. Brackenbury1,2* 4 1Department of Biology, University of York, Heslington, York, YO10 5DD, UK 5 2York Biomedical Research Institute, University of York, Heslington, York, YO10 5DD, UK 6 * Correspondence: Dr William J. Brackenbury, Department of Biology and York Biomedical 7 Research Institute, University of York, Wentworth Way, Heslington, York YO10 5DD, UK. Email: 8 [email protected]. Tel: +44 1904 328284. 9 Keywords: Anticonvulsant, cancer, epilepsy, eslicarbazepine acetate, Nav1.5, S-licarbazepine, 10 voltage-gated Na+ channel. 11 Abstract 12 Eslicarbazepine acetate (ESL) is a dibenzazepine anticonvulsant approved as adjunctive treatment for 13 partial-onset epileptic seizures. Following first pass hydrolysis of ESL, S-licarbazepine (S-Lic) 14 represents around 95 % of circulating active metabolites. S-Lic is the main enantiomer responsible 15 for anticonvulsant activity and this is proposed to be through the blockade of voltage-gated Na+ 16 channels (VGSCs). ESL and S-Lic both have a voltage-dependent inhibitory effect on the Na+ current 17 in N1E-115 neuroblastoma cells expressing neuronal VGSC subtypes including Nav1.1, Nav1.2, 18 Nav1.3, Nav1.6 and Nav1.7. -

Efficacy of Dosulepin in Tension Type Headache and Midfacial Pain a Randomised Controlled Study
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 19, Issue 3 Ser.4 (March. 2020), PP 10-14 www.iosrjournals.org Efficacy of Dosulepin in Tension Type Headache and Midfacial Pain a Randomised Controlled Study Corresponding Author: XXXXX Abstract Introduction: Patients presenting with pain in the facial region are commonly reffered to Otorhinolaryngologist. Due to the various causative factors causing pain in the facial region, the proper diagnosis and more importantly the skill to rule out the sinogenic pain plays an important role in management. Tension type headache and midfacial pain are often misdiagnosed and treated wrongly and thus add to the morbidity of patients. Of all the medications that are used for the treatment, most provide minimal, short term relief with side effects on prolong use. Dosulepin a Tricyclic Antidepressant helps in relieving pain with minimal side effects as compared to other Tricyclic Antidepressants. Aims and Objective: To study the efficacy of dosulepin in patients attending outpatient Department of otorhinolaryngology of Silchar Medical College and Hospital Methods: 130 patients attending outpatient Department of ENT and Head and Neck Surgery of Silchar Medical College & Hospital, from September 2017 to August 2018 with complaints of headache diagnosed as tension type headache and midfacial pain were evaluated. Neurological and radiographic examinations were performed prior to therapy and local cause of pain in the facial region were ruled out. Together with this we also performed psychic evaluation for those patients who could not be withdrawn from current medication and then to start them on our current medication. -

Eslicarbazepine Acetate: a New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures
Drugs R D DOI 10.1007/s40268-017-0197-5 REVIEW ARTICLE Eslicarbazepine Acetate: A New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures 1 1 1 Graciana L. Galiana • Angela C. Gauthier • Richard H. Mattson Ó The Author(s) 2017. This article is an open access publication Abstract Eslicarbazepine acetate is a new anti-epileptic drug belonging to the dibenzazepine carboxamide family Key Points that is currently approved as adjunctive therapy and monotherapy for partial-onset (focal) seizures. The drug Eslicarbazepine acetate is an effective and safe enhances slow inactivation of voltage-gated sodium chan- treatment option for partial-onset seizures as nels and subsequently reduces the activity of rapidly firing adjunctive therapy and monotherapy. neurons. Eslicarbazepine acetate has few, but some, drug– drug interactions. It is a weak enzyme inducer and it Eslicarbazepine acetate improves upon its inhibits cytochrome P450 2C19, but it affects a smaller predecessors, carbamazepine and oxcarbazepine, by assortment of enzymes than carbamazepine. Clinical being available in a once-daily regimen, interacting studies using eslicarbazepine acetate as adjunctive treat- with a smaller range of drugs, and causing less side ment or monotherapy have demonstrated its efficacy in effects. patients with refractory or newly diagnosed focal seizures. The drug is generally well tolerated, and the most common side effects include dizziness, headache, and diplopia. One of the greatest strengths of eslicarbazepine acetate is its ability to be administered only once per day. Eslicar- 1 Introduction bazepine acetate has many advantages over older anti- epileptic drugs, and it should be strongly considered when Epilepsy is a common neurological disorder affecting over treating patients with partial-onset epilepsy. -
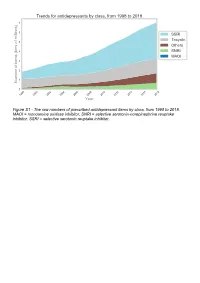
The Raw Numbers of Prescribed Antidepressant Items by Class, from 1998 to 2018
Figure S1 - The raw numbers of prescribed antidepressant items by class, from 1998 to 2018. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Figure S2 - The raw numbers of prescribed antidepressant items for the ten most commonly prescribed antidepressants (in 2018), from 1998 to 2018. Figure S3 - Practice level percentile charts for the proportions of the ten most commonly prescribed antidepressants (in 2018), prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S4 - Practice level percentile charts for the proportions of specific MAOIs prescribed between August 2010 and November 2019. Deciles are in blue, with median shown as a heavy blue line, and extreme percentiles are in red. Figure S5 - CCG level heat map for the percentage proportions of MAOIs prescribed between December 2018 and November 2019. MAOI = monoamine oxidase inhibitor. Figure S6 - CCG level heat maps for the percentage proportions of paroxetine, dosulepin, and trimipramine prescribed between December 2018 and November 2019. Table S1 - Antidepressant drugs, by category. MAOI = monoamine oxidase inhibitor, SNRI = selective serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor. Class Drug MAOI Isocarboxazid, moclobemide, phenelzine, tranylcypromine SNRI Duloxetine, venlafaxine SSRI Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, -

NORPRAMIN® (Desipramine Hydrochloride Tablets USP)
NORPRAMIN® (desipramine hydrochloride tablets USP) Suicidality and Antidepressant Drugs Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of NORPRAMIN or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. NORPRAMIN is not approved for use in pediatric patients. (See WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use.) DESCRIPTION NORPRAMIN® (desipramine hydrochloride USP) is an antidepressant drug of the tricyclic type, and is chemically: 5H-Dibenz[bƒ]azepine-5-propanamine,10,11-dihydro-N-methyl-, monohydrochloride. 1 Reference ID: 3536021 Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: acacia, calcium carbonate, corn starch, D&C Red No. 30 and D&C Yellow No. 10 (except 10 mg and 150 mg), FD&C Blue No. 1 (except 25 mg, 75 mg, and 100 mg), hydrogenated soy oil, iron oxide, light mineral oil, magnesium stearate, mannitol, polyethylene glycol 8000, pregelatinized corn starch, sodium benzoate (except 150 mg), sucrose, talc, titanium dioxide, and other ingredients. -

Fatal Toxicity of Antidepressant Drugs in Overdose
BRITISH MEDICAL JOURNAL VOLUME 295 24 OCTOBER 1987 1021 Br Med J (Clin Res Ed): first published as 10.1136/bmj.295.6605.1021 on 24 October 1987. Downloaded from PAPERS AND SHORT REPORTS Fatal toxicity of antidepressant drugs in overdose SIMON CASSIDY, JOHN HENRY Abstract dangerous in overdose, thus meriting investigation of their toxic properties and closer consideration of the circumstances in which A fatal toxicity index (deaths per million National Health Service they are prescribed. Recommendations may thus be made that prescriptions) was calculated for antidepressant drugs on sale might reduce the number offatalities. during the years 1975-84 in England, Wales, and Scotland. The We used national mortality statistics and prescription data tricyclic drugs introduced before 1970 had a higher index than the to compile fatal toxicity indices for the currently available anti- mean for all the drugs studied (p<0-001). In this group the depressant drugs to assess the comparative safety of the different toxicity ofamitriptyline, dibenzepin, desipramine, and dothiepin antidepressant drugs from an epidemiological standpoint. Owing to was significantly higher, while that ofclomipramine, imipramine, the nature of the disease these drugs are particularly likely to be iprindole, protriptyline, and trimipramine was lower. The mono- taken in overdose and often cause death. amine oxidase inhibitors had intermediate toxicity, and the antidepressants introduced since 1973, considered as a group, had significantly lower toxicity than the mean (p<0-001). Ofthese newer drugs, maprotiline had a fatal toxicity index similar to that Sources ofinformation and methods of the older tricyclic antidepressants, while the other newly The statistical sources used list drugs under their generic and proprietary http://www.bmj.com/ introduced drugs had lower toxicity indices, with those for names. -

Low-Dose Doxepin for Treatment of Pruritus in Patients on Hemodialysis
DIALYSIS Low-Dose Doxepin for Treatment of Pruritus in Patients on Hemodialysis Fatemeh Pour-Reza-Gholi,1 Alireza Nasrollahi,2 Ahmad Firouzan,1 Ensieh Nasli Esfahani,1 Farhat Farrokhi3 1Department of Nephrology, Introduction. Pruritus is one of the frequent discomforting Shaheed Labbafinejad complications in patients with end-stage renal disease. We Medical Center & Urology and prospectively evaluated the effectiveness of doxepin, an H1-receptor Nephrology Research Center, antagonist of histamine, in patients with pruritus resistant to Shaheed Beheshti Medical University, Tehran, Iran conventional treatment. 2Department of Nephrology, Materials and Methods. A randomized controlled trial with a Shohada-e-Tajrish Hospital, crossover design was performed on 24 patients in whom other Shaheed Beheshti Medical etiologic factors of pruritus had been ruled out. They were assigned University, Tehran, Iran into 2 groups and received either placebo or oral doxepin, 10 mg, 3Urology and Nephrology Research Center, Shaheed twice a day for 1 week. After a 1-week washout period, the 2 groups Beheshti Medical University, were treated conversely. Subjective outcome was determined by Tehran, Iran asking the patients described their pruritus as completely improved, relatively improved, or remained unchanged/worsened. Keywords. pruritus, doxepin, Results. Complete resolution of pruritus was reported in end-stage renal disease, 14 patients (58.3%) with doxepin and 2 (8.3%) with placebo dialysis (P < .001). Relative improvement was observed in 7 (29.2%) and 4 (16.7%), respectively. Overall, the improving effect of doxepin on Original Paper pruritus was seen in 87.5% of the patients. Twelve patients (50.0%) complained of drowsiness that alleviated in all cases after 2 days in average. -

Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice
pharmaceuticals Article Doxepin Exacerbates Renal Damage, Glucose Intolerance, Nonalcoholic Fatty Liver Disease, and Urinary Chromium Loss in Obese Mice Geng-Ruei Chang 1,* , Po-Hsun Hou 2,3, Wei-Cheng Yang 4, Chao-Min Wang 1 , Pei-Shan Fan 1, Huei-Jyuan Liao 1 and To-Pang Chen 5,* 1 Department of Veterinary Medicine, National Chiayi University, 580 Xinmin Road, Chiayi 60054, Taiwan; [email protected] (C.-M.W.); [email protected] (P.-S.F.); [email protected] (H.-J.L.) 2 Department of Psychiatry, Taichung Veterans General Hospital, 1650 Taiwan Boulevard (Section 4), Taichung 40705, Taiwan; [email protected] 3 Faculty of Medicine, National Yang-Ming University, 155 Linong Street (Section 2), Taipei 11221, Taiwan 4 School of Veterinary Medicine, National Taiwan University, 1 Roosevelt Road (Section 4), Taipei 10617, Taiwan; [email protected] 5 Division of Endocrinology and Metabolism, Show Chwan Memorial Hospital, 542 Chung-Shan Road (Section 1), Changhua 50008, Taiwan * Correspondence: [email protected] (G.-R.C.); [email protected] (T.-P.C.); Tel.: +886-5-2732946 (G.-R.C.); +886-4-7256166 (T.-P.C.) Abstract: Doxepin is commonly prescribed for depression and anxiety treatment. Doxepin-related disruptions to metabolism and renal/hepatic adverse effects remain unclear; thus, the underlying mechanism of action warrants further research. Here, we investigated how doxepin affects lipid Citation: Chang, G.-R.; Hou, P.-H.; change, glucose homeostasis, chromium (Cr) distribution, renal impairment, liver damage, and fatty Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; liver scores in C57BL6/J mice subjected to a high-fat diet and 5 mg/kg/day doxepin treatment for Liao, H.-J.; Chen, T.-P. -

Heat Related Illness in Psychotropic Medication Users
Common psychotropic medications which Prevention of Heat can impair your response to heat Related Illness Trade Name Generic Name Abilify aripiprazole During periods of high temperature (85º Asendin amoxapine and above) and humidity, there are things Artane trihexyphenidyl everyone, particularly people at high risk, Aventil, Pamelor nortriptyline should do to lessen the chances of heat Clozaril clozapine illness. Cogentin benztropine Compazine prochlorperazine ¾ Try to stay cool. Desyrel trazodone • Stay in air conditioned areas if Elavil, Limbitrol, possible. If you do not have air Triavil amitriptyline conditioning at home, go to a Eskalith, Lithobid, shopping mall or public library. Lithonate lithium • Keep windows shut and draperies, Geodon ziprasidone shades, or blinds drawn during the Haldol haloperidol heat of the day. Loxitane loxapine • Open windows in the evening or Ludiomil maprotiline night hours when the air outside is Mellaril thioridazine Heat Related Illness cooler. Moban molindone • Move to cooler rooms during the Navane thiothixene in heat of the day. Norpramin desipramine Psychotropic ¾ Avoid overexertion and outdoor Phenergan promethazine activity, particularly during warmer Prolixin fluphenazine Medication Users periods of the day. Risperdal risperidone ¾ Apply sunscreen and lotion as needed. Serentil mesoridazine Seroquel quetiapine ¾ Drink plenty of fluids (avoid coffee, tea, and alcohol). Sinequan doxepin ¾ Dress in loose fitting, light colored Stelazine trifluoperazine clothing. Wear a hat, sunglasses, and Thorazine chlorpromazine other protective clothing. Tofranil imipramine ¾ Take a cool shower or bath. Trilafon perphenazine ¾ Lose weight if you are overweight. Wellbutrin buproprion ¾ Eat regular meals to ensure that you Zyprexa olanzapine Ohio Department of Mental Health have adequate salt and fluids. *Note: This is not an all inclusive list.