In Norway: Exploring the Population Decline Through Bird Ringing Data and Habitat Selection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wattled Plovers Arrived to Breed at Carolina During September, Left During March (Little 1967), and the Timing Suggests That They Move to Zimbabwe
400 Charadriidae: plovers be caused by seasonal changes in habitat quality, mostly the availability of short-grass habitat near water. Cold winters at high altitudes with heavy frosts may reduce prey levels to the extent that birds are forced to move. Numbers in Zimbabwe increase during the period late-March to August (Tree 1977). Wattled Plovers arrived to breed at Carolina during September, left during March (Little 1967), and the timing suggests that they move to Zimbabwe. In Zambia there is consider- able movement out of the country during the rains when habitat becomes flooded and overgrown, and it is likely to move to the Caprivi Strip and Okavango Delta (Tree 1969; Aspinwall 1986). Little (1967) found that birds were already in pairs when they arrived and that these pairs were philopatric. Breeding: The season is September–January, with most breeding recorded October–November. The nest site is usually in open grassland, with good visibility. It is highly territorial during the breeding season, excluding conspecifics and many other bird species from its territory which can be large (3–6 ha) Wattled Plover and does not necessarily include the nest site (Little 1967). Lelkiewiet Interspecific relationships: It does not breed within the habitat of any other plover and does not appear to compete Vanellus senegallus with them. It sometimes feeds in loose association with Black- smith V. armatus, Crowned V. coronatus, Blackwinged V. The Wattled Plover occurs widely in sub-Saharan Africa, but melanopterus and Lesser Blackwinged V. lugubris Plovers, is absent from tropical rainforest and arid regions in the north- and Temminck’s Courser Cursorius temminckii (Ward east and southwest. -

Spur-Winged Lapwing Vanellus Spinosus
Spur-winged Lapwing Vanellus spinosus Class: Aves Order: Charadriiformes Family: Charadriidae Characteristics: Also known as the spur-winged plover (not to be confused with the recently renamed masked lapwing of Australasia), this lapwing is a wading bird identified by their striking white cheek feathers, black head cap, brown wings against a black body and long black legs. Behavior: In Africa, lapwings don’t travel far outside their home area but merely make short movements to find wetter areas of their habitats. They spend Range & Habitat: their time searching the marshy ground for small invertebrates. Marshes and wetland habitats of central Africa Reproduction: Because of their large range, these birds have variable breeding seasons. Spur-winged lapwings nest in solitary monogamous pairs, often with other mixed species bird nesting colonies. The large nesting groups help protect the birds in the colonies against predation. The lapwing pair will build a nest in a scrape on the ground sometimes lined with vegetation. The female lays 2 eggs that are yellow with brownish black mottling. They hatch after a 28-day incubation period and both sexes help feed the young. If they double-clutch, the male tends the older chicks while the female incubates the second brood (Sacramento Zoo). Lifespan: over 15 years in Diet: captivity, up to 15 years in the Wild: Invertebrates wild. Zoo: softbill, feline diet, capelin, mealworms and insectivore diet Special Adaptations: Spur- Conservation: winged lapwings have a unique Spur-winged lapwings are abundant in their range in Africa and as such call that acts as an alert when are listed as Least Concern by IUCN. -

Developing Methods for the Field Survey and Monitoring of Breeding Short-Eared Owls (Asio Flammeus) in the UK: Final Report from Pilot Fieldwork in 2006 and 2007
BTO Research Report No. 496 Developing methods for the field survey and monitoring of breeding Short-eared owls (Asio flammeus) in the UK: Final report from pilot fieldwork in 2006 and 2007 A report to Scottish Natural Heritage Ref: 14652 Authors John Calladine, Graeme Garner and Chris Wernham February 2008 BTO Scotland School of Biological and Environmental Sciences, University of Stirling, Stirling, FK9 4LA Registered Charity No. SC039193 ii CONTENTS LIST OF TABLES................................................................................................................... iii LIST OF FIGURES ...................................................................................................................v LIST OF FIGURES ...................................................................................................................v LIST OF APPENDICES...........................................................................................................vi SUMMARY.............................................................................................................................vii EXECUTIVE SUMMARY ................................................................................................... viii CRYNODEB............................................................................................................................xii ACKNOWLEDGEMENTS....................................................................................................xvi 1. BACKGROUND AND AIMS...........................................................................................2 -
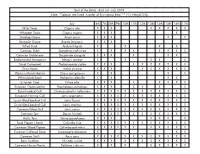
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Appendix A. Supplementary Material
Appendix A. Supplementary material Comprehensive taxon sampling and vetted fossils help clarify the time tree of shorebirds (Aves, Charadriiformes) David Cernˇ y´ 1,* & Rossy Natale2 1Department of the Geophysical Sciences, University of Chicago, Chicago 60637, USA 2Department of Organismal Biology & Anatomy, University of Chicago, Chicago 60637, USA *Corresponding Author. Email: [email protected] Contents 1 Fossil Calibrations 2 1.1 Calibrations used . .2 1.2 Rejected calibrations . 22 2 Outgroup sequences 30 2.1 Neornithine outgroups . 33 2.2 Non-neornithine outgroups . 39 3 Supplementary Methods 72 4 Supplementary Figures and Tables 74 5 Image Credits 91 References 99 1 1 Fossil Calibrations 1.1 Calibrations used Calibration 1 Node calibrated. MRCA of Uria aalge and Uria lomvia. Fossil taxon. Uria lomvia (Linnaeus, 1758). Specimen. CASG 71892 (referred specimen; Olson, 2013), California Academy of Sciences, San Francisco, CA, USA. Lower bound. 2.58 Ma. Phylogenetic justification. As in Smith (2015). Age justification. The status of CASG 71892 as the oldest known record of either of the two spp. of Uria was recently confirmed by the review of Watanabe et al. (2016). The younger of the two marine transgressions at the Tolstoi Point corresponds to the Bigbendian transgression (Olson, 2013), which contains the Gauss-Matuyama magnetostratigraphic boundary (Kaufman and Brigham-Grette, 1993). Attempts to date this reversal have been recently reviewed by Ohno et al. (2012); Singer (2014), and Head (2019). In particular, Deino et al. (2006) were able to tightly bracket the age of the reversal using high-precision 40Ar/39Ar dating of two tuffs in normally and reversely magnetized lacustrine sediments from Kenya, obtaining a value of 2.589 ± 0.003 Ma. -

International Journal of Biodiversity Andconservation
OPEN ACCESS International Journal of Biodiversity andConservation February 2019 ISSN 2141-243X DOI: 10.5897/IJBC www.academicjournals.org About IJBC International Journal of Biodiversity and Conservation (IJBC)provides rapid publication (monthly) of articles in all areas of the subject such as Information Technology and its Applications in Environmental Management and Planning, Environmental Management and Technologies, Green Technology and Environmental Conservation, Health: Environment and Sustainable Development etc. The Journal welcomes the submission of manuscripts that meet the general criteria of significance and scientific excellence. Papers will be published shortly after acceptance. All articles published in IJBC are peer reviewed. Indexing The International Journal of Biodiversity and Conservation is indexed in: CAB Abstracts, CABI’s Global Health Database, China National Knowledge Infrastructure (CNKI), Dimensions Database, Google Scholar, Matrix of Information for The Analysis of Journals (MIAR), Microsoft Academic IJBC has an h5-index of 13 on Google Scholar Metrics Open Access Policy Open Access is a publication model that enables the dissemination of research articles to the global community without restriction through the internet. All articles published under open access can be accessed by anyone with internet connection. The International Journal of Biodiversity and Conservation is an Open Access journal. Abstracts and full texts of all articles published in this journal are freely accessible to everyone immediately after publication without any form of restriction. Article License All articles published by International Journal of Biodiversity and Conservation are licensed under the Creative Commons Attribution 4.0 International License. This permits anyone to copy, redistribute, remix, transmit and adapt the work provided the original work and source is appropriately cited. -

Birdwatching in Portugal
birdwatchingIN PORTUGAL In this guide, you will find 36 places of interest 03 - for birdwatchers and seven suggestions of itineraries you may wish to follow. 02 Accept the challenge and venture forth around Portugal in search of our birdlife. birdwatching IN PORTUGAL Published by Turismo de Portugal, with technical support from Sociedade Portuguesa para o Estudo das Aves (SPEA) PHOTOGRAPHY Ana Isabel Fagundes © Andy Hay, rspb-images.com Carlos Cabral Faisca Helder Costa Joaquim Teodósio Pedro Monteiro PLGeraldes SPEA/DLeitão Vitor Maia Gerbrand AM Michielsen TEXT Domingos Leitão Alexandra Lopes Ana Isabel Fagundes Cátia Gouveia Carlos Pereira GRP A HIC DESIGN Terradesign Jangada | PLGeraldes 05 - birdwatching 04 Orphean Warbler, Spanish Sparrow). The coastal strip is the preferred place of migration for thousands of birds from dozens of different species. Hundreds of thousands of sea and coastal birds (gannets, shear- waters, sandpipers, plovers and terns), birds of prey (eagles and harriers), small birds (swallows, pipits, warblers, thrushes and shrikes) cross over our territory twice a year, flying between their breeding grounds in Europe and their winter stays in Africa. ortugal is situated in the Mediterranean region, which is one of the world’s most im- In the archipelagos of the Azores and Madeira, there p portant areas in terms of biodiversity. Its are important colonies of seabirds, such as the Cory’s landscape is very varied, with mountains and plains, Shearwater, Bulwer’s Petrel and Roseate Tern. There are hidden valleys and meadowland, extensive forests also some endemic species on the islands, such as the and groves, rocky coasts and never-ending beaches Madeiran Storm Petrel, Madeiran Laurel Pigeon, Ma- that stretch into the distance, estuaries, river deltas deiran Firecrest or the Azores Bullfinch. -

The Origin Oflapwings Vanellus Vanellus and Golden Plovers
Ornis Fennica 81 :49-64. 2004 The origin of Lapwings Vanellus vanellus and Golden Plovers Pluvialis apricaria wintering in Portugal Domingos Leitão & Salvador Peris Leitão, D., Centro deBiología Ambiental, Departamento de BiologíaAnimal, Faculdade de Ciéncias da Universidade de Lisboa, Edificio C2, Campo Grande, 1700 Lisboa, Por- tugal. Present address: Sociedade Portuguesa para o Estudo das Aves, Rua da Vitória, 53, 3~Esq, 1100-618 Lisboa, Portugal, e-mail: [email protected] Peris, S., Departamento de Biología Animal, Facultad de Biología, Universidad de Salamanca, E-37071, Salamanca, Spain Received 2 December 2003, accepted 22 March 2004 Lapwing (Vanellus vanellus) and Golden Plover (Pluvialis apricaria) ring recoveries in Portugal were compared in order to search for trends in their origin, to analyse the rela- tionship between cold weather and the wintering population, and to discuss the age pat- tern of the birds. Most Lapwings wintering in Portugal come from the British Isles, the Netherlands, Southern ScandinavianPeninsula and Denmark, and Germany. Golden Plo- vers come from all breeding range, a vast region extending from Iceland to Siberia. Colder temperatures in mid-Winter in Northwest Europe bring more birds ofboth species to Por- tugal. After the late 1960s and during 1970s, a systematic decrease occurred inthe Recov- ery Index ofLapwing andGolden Plover coming from several countries in North-western Europe . In the 1980s this trend persisted for birds coming from Scandinavian countries and was inverted for more southern countries. Changes in winter weather could be the most important factor determining these patterns. When winters are mild thebirds may be able to remain longer in northern and eastern areas that have previously been unsuitable. -

Foxes, Voles, and Waders: Drivers of Predator Activity in Wet Grassland Landscapes
VOLUME 14, ISSUE 2, ARTICLE 4 Laidlaw, R. A., J. Smart, M. A. Smart, T. W. Bodey, T. Coledale, and J. A. Gill. 2019. Foxes, voles, and waders: drivers of predator activity in wet grassland landscapes. Avian Conservation and Ecology 14(2):4. https://doi.org/10.5751/ACE-01414-140204 Copyright © 2019 by the author(s). Published here under license by the Resilience Alliance. Research Paper Foxes, voles, and waders: drivers of predator activity in wet grassland landscapes Rebecca A. Laidlaw 1, Jennifer Smart 1,2, Mark A. Smart 3, Thomas W. Bodey 2,4, Tessa Coledale 5 and Jennifer A. Gill 1 1School of Biological Sciences, University of East Anglia, Norwich Research Park, Norwich, UK, 2RSPB Centre for Conservation Science, Royal Society for the Protection of Birds, The Lodge, Sandy, UK, 3Royal Society for the Protection of Birds, The Lodge, Sandy, UK, 4Environment & Sustainability Institute, University of Exeter Penryn Campus, Penryn, UK, 5Royal Society for the Protection of Birds, Scotland Headquarters, Edinburgh, UK ABSTRACT. Impacts of generalist predators on declining prey populations are a major conservation issue, but management of this situation is constrained by limited knowledge of the factors influencing predator distribution and activity. In many declining populations of ground-nesting waders, high levels of nest and chick predation are preventing population recovery. Red foxes, Vulpes vulpes, are the main predator but their primary prey is small mammals. On wet grasslands managed for breeding waders, small mammals are concentrated in tall vegetation outside of fields, and nests closer to these patches are less likely to be predated. -

Grey-Headed Lapwing Keri (Jpn) Vanellus Cinereus Morphology and Classification Breeding System: Grey-Headed Lapwings Are Monogamous
Bird Research News Vol.5 No.11 2008.11.16. Grey-Headed Lapwing Keri (Jpn) Vanellus cinereus Morphology and classification Breeding system: Grey-headed Lapwings are monogamous. They breed from March Classification: Charadriiformes Charadriidae to August. A pair hold a 2.1ha territory on average and defend it together. It is unknown whether the male and female build a nest Total length: ♂ 341.7 ± 12.1mm (n=14) ♀ 336.3 ± 12.1mm (12) together, but both sexes incubate the eggs alternately. The hatch- Wing length: ♂ 236.9 ± 7.5mm (14) ♀ 235.5 ± 4.6mm (12) lings soon leave the nest and start to forage for food by themselves Tail length: ♂ 109.4 ± 3.7mm (14) ♀ 109.8 ± 2.8mm (11) following their parent birds in the territory. Parent birds incubate Culmen length: ♂ 42.02 ± 2.91mm (14) ♀ 41.37 ± 3.00mm (12) and defend their hatchlings if necessary. When the young learn to Tarsus length: ♂ 76.90 ± 2.70mm (14) ♀ 76.26 ± 3.45mm (12) Wing claw: ♂ 5.08 ± 1.35mm (14) ♀ 3.69 ± 0.54mm (12) fly, the family flock leaves the territory. They usually breed once Weight: ♂ 280.1 ± 15.9g (13) ♀ 266.6 ± 19.7g (10) in a breeding season, and the second breeding is rare. When they failed in the first breeding attempt, however, they try to re-nest up Measurements after Wakisaka et al. (2006). to two times (Takahashi 2007). Appearance: Nest: Males and females are similar in They build a nest in wet habitats, plumage coloration. Adult birds are such as paddy fields and low grass bluish gray from the head to the and bare areas around them, such upper chest with a black band on the as ridges, cropland, fallow fields chest. -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Alpha Codes for 2168 Bird Species (And 113 Non-Species Taxa) in Accordance with the 62Nd AOU Supplement (2021), Sorted Taxonomically
Four-letter (English Name) and Six-letter (Scientific Name) Alpha Codes for 2168 Bird Species (and 113 Non-Species Taxa) in accordance with the 62nd AOU Supplement (2021), sorted taxonomically Prepared by Peter Pyle and David F. DeSante The Institute for Bird Populations www.birdpop.org ENGLISH NAME 4-LETTER CODE SCIENTIFIC NAME 6-LETTER CODE Highland Tinamou HITI Nothocercus bonapartei NOTBON Great Tinamou GRTI Tinamus major TINMAJ Little Tinamou LITI Crypturellus soui CRYSOU Thicket Tinamou THTI Crypturellus cinnamomeus CRYCIN Slaty-breasted Tinamou SBTI Crypturellus boucardi CRYBOU Choco Tinamou CHTI Crypturellus kerriae CRYKER White-faced Whistling-Duck WFWD Dendrocygna viduata DENVID Black-bellied Whistling-Duck BBWD Dendrocygna autumnalis DENAUT West Indian Whistling-Duck WIWD Dendrocygna arborea DENARB Fulvous Whistling-Duck FUWD Dendrocygna bicolor DENBIC Emperor Goose EMGO Anser canagicus ANSCAN Snow Goose SNGO Anser caerulescens ANSCAE + Lesser Snow Goose White-morph LSGW Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Intermediate-morph LSGI Anser caerulescens caerulescens ANSCCA + Lesser Snow Goose Blue-morph LSGB Anser caerulescens caerulescens ANSCCA + Greater Snow Goose White-morph GSGW Anser caerulescens atlantica ANSCAT + Greater Snow Goose Intermediate-morph GSGI Anser caerulescens atlantica ANSCAT + Greater Snow Goose Blue-morph GSGB Anser caerulescens atlantica ANSCAT + Snow X Ross's Goose Hybrid SRGH Anser caerulescens x rossii ANSCAR + Snow/Ross's Goose SRGO Anser caerulescens/rossii ANSCRO Ross's Goose