Morphological Study of the Labial Sensilla in Nabidae (Hemiptera: Heteroptera: Cimicomorpha)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taber's Cyclopedic Medical Dictionary
#49016 - Venes: Taber’s Cyclopedia Medical Dictionary -- 21st Edition -- FADavis B  (ba¯Јta˘) Beta, second letter of the Greek protected from tick exposure. Asplenic alphabet. SEE: beta. persons should avoid endemic areas. Af- -glucan (ba¯Јta˘-glooЈka˘n) Any one of a ter possible exposure, removal of ticks class of complexcarbohydrate nutri- or their nymphs may prevent infection. ents, derived from yeast, with immune- TREATMENT: Drugs used include stimulating and antimicrobial activity atovaquone and quinine plus clinda- in laboratory experiments. They are mycin or azithromycin, both given promoted as dietary supplements. orally. Asplenic patients may require -glucuronidase (ba¯Јta˘-glooЉku¯-ro˘nЈ˘-ı exchange transfusion. da¯s) An enzyme found in lysosomes. It Babinski’s reflex (ba˘-bı˘nЈske¯z) [Joseph is involved in the breakdown of glycos- Babinski, Fr. neurologist, 1857–1932] aminoglycan. Dorsiflexion of the great toe when the B 1. Symbol for the element boron. 2. Ba- sole of the foot is stimulated. Normally, cillus; Balantidium; barometric; base; when the lateral aspect of the sole of the bath; behavior; buccal. relaxed foot is stroked, the great toe Ba Symbol for the element barium. flexes. If the toe extends instead of BAAM Beck airway airflow monitor. flexes and the outer toes spread out, Ba- Babcock’s operation (ba˘bЈko˘ks) [Wil- binski’s reflexis present. It is a normal liam Wayne Babcock, U.S. surgeon, reflexin infants under the age of 6 1872–1963] Extirpation of the saphe- months but indicates a lesion of the py- nous vein; a treatment for varicose ramidal (corticospinal) tract in older in- veins. -

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

(Pentatomidae) DISSERTATION Presented
Genome Evolution During Development of Symbiosis in Extracellular Mutualists of Stink Bugs (Pentatomidae) DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Alejandro Otero-Bravo Graduate Program in Evolution, Ecology and Organismal Biology The Ohio State University 2020 Dissertation Committee: Zakee L. Sabree, Advisor Rachelle Adams Norman Johnson Laura Kubatko Copyrighted by Alejandro Otero-Bravo 2020 Abstract Nutritional symbioses between bacteria and insects are prevalent, diverse, and have allowed insects to expand their feeding strategies and niches. It has been well characterized that long-term insect-bacterial mutualisms cause genome reduction resulting in extremely small genomes, some even approaching sizes more similar to organelles than bacteria. While several symbioses have been described, each provides a limited view of a single or few stages of the process of reduction and the minority of these are of extracellular symbionts. This dissertation aims to address the knowledge gap in the genome evolution of extracellular insect symbionts using the stink bug – Pantoea system. Specifically, how do these symbionts genomes evolve and differ from their free- living or intracellular counterparts? In the introduction, we review the literature on extracellular symbionts of stink bugs and explore the characteristics of this system that make it valuable for the study of symbiosis. We find that stink bug symbiont genomes are very valuable for the study of genome evolution due not only to their biphasic lifestyle, but also to the degree of coevolution with their hosts. i In Chapter 1 we investigate one of the traits associated with genome reduction, high mutation rates, for Candidatus ‘Pantoea carbekii’ the symbiont of the economically important pest insect Halyomorpha halys, the brown marmorated stink bug, and evaluate its potential for elucidating host distribution, an analysis which has been successfully used with other intracellular symbionts. -

Good Water Ripples Volume 7 Number 4
For information contact: http://txmn.org/goodwater [email protected] Volume 7 Number 4 August/September 2018 Editor: Mary Ann Melton Fall Training Class Starts Soon Good Water Mas- ter Naturalist Fall Training Class will start Tuesday even- ing, September 4th. The class will meet UPCOMING EVENTS on Tuesday eve- nings from 6:00- 8/9/18 NPSOT 9:30 p.m. Some 8/13/18 WAG classes and field trips will be on Sat- 8/23/18 GWMN urdays. The first class is Tuesday, Austin Butterfly Forum 8/27/18 September 4. The 9/5/18 NPAT last class will be December 11. Cost is $150 and includes the comprehensive Texas Master 9/13/18 NPSOT Naturalist Program manual as well as a one year membership to the Good 9/20/18 Travis Audubon Water Chapter. For couples who plan to share the manual, there is a dis- count for the second student. 9/24/18 Austin Butterfly Forum Click here for online registration. The Tuesday classes will start at 6:00 9/27/18 GWMN p.m. and finish around 9:30. There are four Saturday field trips and classes planned. The schedule will be posted in the next week or so. Check back Check the website for additional here after August 15 for the link to the schedule. events including volunteer and training opportunities. The events Click here: https://txmn.org/goodwater/Training-class-online-application/ are too numerous to post here. for Online Training Registration David Robinson took our Spring Training Class this year. He says, "The Fall Training Class Starts Soon 1 Instructors & Speakers were absolutely fantastic. -

The Genus Pagasa (Hemiptera: Heteroptera: Nabidae: Prostemmatinae) in Mexico, New Records and Key to the Known Species
Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 89 (2018): 1060 - 1067 Taxonomy and systematics The genus Pagasa (Hemiptera: Heteroptera: Nabidae: Prostemmatinae) in Mexico, new records and key to the known species El género Pagasa (Hemiptera: Heteroptera: Nabidae: Prostemmatinae) en México, registros nuevos y clave para las especies conocidas Harry Brailovsky * y Ernesto Barrera Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70153, 04510 Ciudad de México, Mexico *Corresponding author: [email protected] (H. Brailovsky) Received: 10 November 2017; accepted: 19 June 2018 Abstract The genus Pagasa Stål (Heteroptera: Nabidae: Prostemmatinae) from Mexico is revised. Pagasa (P.) henryi Kerzhner is recorded for the first time, and new records to P. (L.) confusa Kerzhner, P. (L.) fusca (Stein), P. (P.) brailovskyi Kerzhner, P. (P.) luteiceps (Walker), P. (P.) pallipes (Stål) and P. (P.) prostemmatoides Kerzhner are given. Key to subgenera and species is added; notes on the biology of some species are given, and photographs of dorsal habitus and parameres are provided. Keywords: Insecta; Damsel bugs; Pagasa; Distribution; Mexico Resumen Se revisa el género Pagasa Stål (Heteroptera: Nabidae: Prostemmatinae) para Mexico. P. (P). henryi Kerzhner se registra por primera vez para la República Mexicana y se proporcionan nuevos datos distribucionales para P. (L.) confusa Kerzhner, P. (L.) fusca (Stein), P. (P.) brailovskyi Kerzhner, P. (P.) luteiceps (Walker), P. (P.) pallipes (Stål) y P. (P.) prostemmatoides Kerzhner. Se agrega una clave para separar los subgéneros y especies mexicanas; se incluyen algunas notas biológicas, así como fotografías en vista dorsal y de los parámeros. Palabras clave: Insecta; Insectos damisela; Pagasa; Distribución; México Introduction control (Kerzhner & Henry, 2008). -

New Records of Heteroptera (Hemiptera) Species from Turkey, with Reconsideration of Several Previous Records
Use the following type of citation: North-western Journal of Zoology 2021: e201203 Paper Submitted to The North-Western Journal of Zoology 1 *Handling editor: Dr. H. Lotfalizadeh 2 *Manuscript Domain: Entomology 3 *Manuscript code: nwjz-20-EN-HL-07 4 *Submission date: 21 September 2020 5 *Revised: 12 December 2020 6 *Accepted: 12 December 2020 7 *No. of words (without abstract, acknowledgement, references, tables, captions): 7431 8 (papers under 700 words are not accepted) 9 *Editors only: 10 11 Zoology 12 Title of the paper: New records of Heteroptera (Hemiptera)of species from Turkey, with 13 reconsideration of several previous records proofing 14 Journaluntil 15 Running head: New Records of Heteroptera from Turkey 16 paper 17 Authors (First LAST - without institution name!): Barış ÇERÇİ, Serdar TEZCAN 18 North-western 19 Accepted 20 Key Words (at least five keywords): New records, previous records, Nabidae, Reduviidae, Miridae, 21 Turkey 22 23 24 No. of Tables: 0 25 No. of Figures: 4 26 No. of Files (landscape tables should be in separate file): 0 Use the following type of citation: North-western Journal of Zoology 2021: e201203 nwjz-2 27 New records of Heteroptera (Hemiptera) species from Turkey, with reconsideration of 28 several previous records 29 Barış, ÇERÇİ1, Serdar, TEZCAN2 30 1. Faculty of Medicine, Hacettepe University, Ankara, Turkey 31 2. Department of Plant Protection, Faculty of Agriculture, Ege University, Izmir, Turkey 32 * Corresponding authors name and email address: Barış ÇERÇİ, 33 [email protected] 34 35 Abstract. In this study, Acrotelus abbaricus Linnavuori, Dicyphus (Dicyphus) josifovi Rieger, 36 Macrotylus (Macrotylus) soosi Josifov, Myrmecophyes (Myrmecophyes) variabilis Drapulyok Zoology 37 and Paravoruchia dentata Wagner are recorded from Turkey for the first time. -

Hemiptera: Heteroptera: Pentatomoidea
VIVIANA CAUDURO MATESCO SISTEMÁTICA DE THYREOCORIDAE AMYOT & SERVILLE (HEMIPTERA: HETEROPTERA: PENTATOMOIDEA): REVISÃO DE ALKINDUS DISTANT, MORFOLOGIA DO OVO DE DUAS ESPÉCIES DE GALGUPHA AMYOT & SERVILLE E ANÁLISE CLADÍSTICA DE CORIMELAENA WHITE, COM CONSIDERAÇÕES SOBRE A FILOGENIA DE THYREOCORIDAE, E MORFOLOGIA DO OVO DE 16 ESPÉCIES DE PENTATOMIDAE COMO EXEMPLO DO USO DE CARACTERES DE IMATUROS EM FILOGENIAS Tese apresentada ao Programa de Pós-Graduação em Biologia Animal, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, como requisito parcial à obtenção do Título de Doutor em Biologia Animal. Área de concentração: Biologia Comparada Orientadora: Profa. Dra. Jocelia Grazia Co-Orientador: Prof. Dr. Cristiano F. Schwertner UNIVERSIDADE FEDERAL DO RIO GRANDE DO SUL PORTO ALEGRE 2014 “Sistemática de Thyreocoridae Amyot & Serville (Hemiptera: Heteroptera: Pentatomoidea): revisão de Alkindus Distant, morfologia do ovo de duas espécies de Galgupha Amyot & Serville e análise cladística de Corimelaena White, com considerações sobre a filogenia de Thyreocoridae, e morfologia do ovo de 16 espécies de Pentatomidae como exemplo de uso de caracteres de imaturos em filogenias” VIVIANA CAUDURO MATESCO Tese apresentada como parte dos requisitos para obtenção de grau de Doutor em Biologia Animal, área de concentração Biologia Comparada. ________________________________________ Prof. Dr. Augusto Ferrari (UFRGS) ________________________________________ Dra. Caroline Greve (CNPq ex-bolsista PDJ) ________________________________________ Prof. Dr. Cláudio José Barros de Carvalho (UFPR) ________________________________________ Profa. Dra. Jocelia Grazia (Orientadora) Porto Alegre, 05 de fevereiro de 2014. AGRADECIMENTOS À minha orientadora, Profa. Dra. Jocelia Grazia, pelos ensinamentos e por todas as oportunidades que me deu durante os treze anos em que estive no Laboratório de Entomologia Sistemática. Ao meu co-orientador, Prof. -

Descriptions.-Collyer, 1953 ; Fubnek, 1930; Hall, 1951; Leston, 1954A; Peska, 1931; Poisson, 1933; Sands, 1954; Swezey, 1905
the eggs of the terrestrial Hetroptera 205 or stems of their host. The eggs of species living in stored products are laid, like those of (Jimex, in exposed or semi-exposed situations. , The eggs of the Anthocoridae show a general similarity to those of the Cimicidae, but differ in the presence ofa reduced network region. This character is also found in the Nabidae and Microphysidae. The thickened operculum of Acompocons is similar to that inthe Nabidae, but the elliptical-shaped operculum of EBltophilus is found elsewhere only in the Miridae. Descriptions.-Collyer, 1953 ; Fubnek, 1930; Hall, 1951; Leston, 1954a; Peska, 1931; Poisson, 1933; Sands, 1954; Swezey, 1905. Microphysidae. Only the eggs of Loriula and Myrmedobia have been described. These are very characteristic with tapering processes that probably correspond to the network region (fig. 6N) (Carayon, 1949b). The eggs of Loricula elegantula (Barensprung) *xe very largecompared with the adultfemale, two (the maximum number found) almost filling the abdomen. These ovarian eggs have been studied and the pseudoinicropyles found to be short, as in the Anthocoridae, terminating at the base of the network region. In the ovarian egg the processes of the network region touch at their tips and thus cover the operculum, but Carayon (1949b) found that in the laid egg they radiate out and thus their apices become widely separate. The operculum is thin and flat with a pattern, due to the follicle cells, similar to that of the Anthocoridae. (Carayon (1949b) found the eggs, that are grey to brown in colour, inserted amongst lichens growing on the twigs of various trees. -

A Comparison of the External Morphology and Functions of Labial Tip Sensilla in Semiaquatic Bugs (Hemiptera: Heteroptera: Gerromorpha)
Eur. J. Entomol. 111(2): 275–297, 2014 doi: 10.14411/eje.2014.033 ISSN 1210-5759 (print), 1802-8829 (online) A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) 1 2 JOLANTA BROŻeK and HERBERT ZeTTeL 1 Department of Zoology, Faculty of Biology and environmental Protection, University of Silesia, Bankowa 9, PL 40-007 Katowice, Poland; e-mail: [email protected] 2 Natural History Museum, entomological Department, Burgring 7, 1010 Vienna, Austria; e-mail: [email protected] Key words. Heteroptera, Gerromorpha, labial tip sensilla, pattern, morphology, function, apomorphic characters Abstract. The present study provides new data on the morphology and distribution of the labial tip sensilla of 41 species of 20 gerro- morphan (sub)families (Heteroptera: Gerromorpha) obtained using a scanning electron microscope. There are eleven morphologically distinct types of sensilla on the tip of the labium: four types of basiconic uniporous sensilla, two types of plate sensilla, one type of peg uniporous sensilla, peg-in-pit sensilla, dome-shaped sensilla, placoid multiporous sensilla and elongated placoid multiporous sub- apical sensilla. Based on their external structure, it is likely that these sensilla are thermo-hygrosensitive, chemosensitive and mechano- chemosensitive. There are three different designs of sensilla in the Gerromorpha: the basic design occurs in Mesoveliidae and Hebridae; the intermediate one is typical of Hydrometridae and Hermatobatidae, and the most specialized design in Macroveliidae, Veliidae and Gerridae. No new synapomorphies for Gerromorpha were identified in terms of the labial tip sensilla, multi-peg structures and shape of the labial tip, but eleven new diagnostic characters are recorded for clades currently recognized in this infraorder. -
Hemiptera, Heteroptera, Miridae, Isometopinae) from Borneo with Remarks on the Distribution of the Tribe
ZooKeys 941: 71–89 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.941.47432 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research Two new genera and species of the Gigantometopini (Hemiptera, Heteroptera, Miridae, Isometopinae) from Borneo with remarks on the distribution of the tribe Artur Taszakowski1*, Junggon Kim2*, Claas Damken3, Rodzay A. Wahab3, Aleksander Herczek1, Sunghoon Jung2,4 1 Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, University of Silesia in Katowice, Bankowa 9, 40-007 Katowice, Poland 2 Laboratory of Systematic Entomology, Depart- ment of Applied Biology, College of Agriculture and Life Sciences, Chungnam National University, Daejeon, South Korea 3 Institute for Biodiversity and Environmental Research, Universiti Brunei Darussalam, Jalan Universiti, BE1410, Darussalam, Brunei 4 Department of Smart Agriculture Systems, College of Agriculture and Life Sciences, Chungnam National University, Daejeon, South Korea Corresponding author: Artur Taszakowski ([email protected]); Sunghoon Jung ([email protected]) Academic editor: F. Konstantinov | Received 21 October 2019 | Accepted 2 May 2020 | Published 16 June 2020 http://zoobank.org/B3C9A4BA-B098-4D73-A60C-240051C72124 Citation: Taszakowski A, Kim J, Damken C, Wahab RA, Herczek A, Jung S (2020) Two new genera and species of the Gigantometopini (Hemiptera, Heteroptera, Miridae, Isometopinae) from Borneo with remarks on the distribution of the tribe. ZooKeys 941: 71–89. https://doi.org/10.3897/zookeys.941.47432 Abstract Two new genera, each represented by a single new species, Planicapitus luteus Taszakowski, Kim & Her- czek, gen. et sp. nov. and Bruneimetopus simulans Taszakowski, Kim & Herczek, gen. et sp. nov., are described from Borneo. -
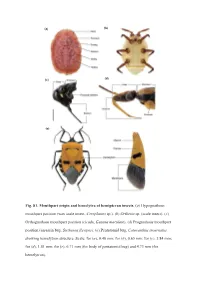
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

First Ever Report of a Bite by Nabis Argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a Human
Short Communication Biodiversity and Natural History (2017) Vol. 3, No. 2, 45-47 First ever report of a bite by Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a human Primer registro de una picadura de Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) en un ser humano Marcela Cornelis1, Fernando Diez1 & María del Carmen Coscarón2 1Universidad Nacional de La Pampa, Facultad de Ciencias Exactas y Naturales, Uruguay 151 L6300CLB, Santa Rosa, La Pampa, Argentina. 2Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo, División Entomología, Paseo del Bosque s/n 1900, La Plata, Buenos Aires, Argentina. *Correspondence author: [email protected] Abstract Herein, we described the first ever reported bite of Nabis argentinus Meyer-Dür 1870 on a human. The bite was registered in the locality Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). The insect was not provoked by the victim, and thus, the bite was probably not in self-defense. We therefore concluded that the insect bite the victim because it was searching for sources of hydration. Key words: adventitious bite, nabids, predaceous habit, record, symptomatology. Resumen Se describe el primer caso de una picadura adventicia en un ser humano por Nabis argentinus Meyer-Dür 1870, en la localidad de Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). El insecto no fue provocado por la víctima, por lo que probablemente la picadura no fue en defensa propia. Por lo tanto, concluimos que el insecto picó a la víctima en búsqueda de recursos de hidratación.