Guidelines on Treatment of Extravasation with Cytotoxic Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MASCC/ESMO ANTIEMETIC GUIDELINE 2016 with Updates in 2019
1 ANTIEMETIC GUIDELINES: MASCC/ESMO MASCC/ESMO ANTIEMETIC GUIDELINE 2016 With Updates in 2019 Organizing and Overall Meeting Chairs: Matti Aapro, MD Richard J. Gralla, MD Jørn Herrstedt, MD, DMSci Alex Molassiotis, RN, PhD Fausto Roila, MD © Multinational Association of Supportive Care in CancerTM All rights reserved worldwide. 2 ANTIEMETIC GUIDELINES: MASCC/ESMO These slides are provided to all by the Multinational Association of Supportive Care in Cancer and can be used freely, provided no changes are made and the MASCC and ESMO logos, as well as date of the information are retained. For questions please contact: Matti Aapro at [email protected] Chair, MASCC Antiemetic Study Group or Alex Molassiotis at [email protected] Past Chair, MASCC Antiemetic Study Group 3 ANTIEMETIC GUIDELINES: MASCC/ESMO Consensus A few comments on this guideline set: • This set of guideline slides represents the latest edition of the guideline process. • This set of slides has been endorsed by the MASCC Antiemetic Guideline Committee and ESMO Guideline Committee. • The guidelines are based on the votes of the panel at the Copenhagen Consensus Conference on Antiemetic Therapy, June 2015. • Latest version: March 2016, with updates in 2019. 4 ANTIEMETIC GUIDELINES: MASCC/ESMO Changes: The Steering Committee has clarified some points: 2016: • A footnote clarified that aprepitant 165 mg is approved by regulatory authorities in some parts of the world ( although no randomised clinical trial has investigated this dose ). Thus use of aprepitant 80 mg in the delayed phase is only for those cases where aprepitant 125 mg is used on day 1. • A probable modification in pediatric guidelines based on the recent Cochrane meta-analysis is indicated. -

Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Acla
[CANCER RESEARCH 49. 4357-4362, August 1. 1989] Transcatheter Arterial Chemoembolization Therapy for Hepatocellular Carcinoma Using Polylactic Acid Microspheres Containing Aclarubicin Hydrochloride 1omonimi Ichihara,1 Kiyoshi Sakamoto, Katsutaka Mori, and Masanobu Akagi Department of Surgery II, Kumamoto University Medical School, Kumamoto 860, Japan 4BSTRACT MATERIALS AND METHODS Transcatheter arterial Chemoembolization therapy using polylactic Preparation of PLA-ACRms acid microspheres containing aclarubicin hydrochloride (ACR) was per PLA-ACRms were prepared in the pharmacy of the Kumamoto formed in 62 patients with primary hepatocellular carcinoma. These microspheres were about 200 ¡anin diameter and contained 10% (w/w) University Hospital. Briefly, aclarubicin hydrochloride and isopropyl aclarubicin. A single dose of polylactic acid microspheres containing ACR myristate, a medium-chain fatty acid ester, were dissolved in 7.5% (50-100 mg of ACR) was administered 1 to 8 times with a mean of 2.2 polylactic acid-methylene chloride. The resultant solution was dispersed in 1% gelatin solution and sterilized in an autoclave for 20 min at doses (a total of 160 treatments) in 62 patients. Antitumor effects were 120°C;thismixture was then stirred with a magnetic stirrer at 500 rpm observed from the decrease in serum a-fetoprotein levels (82.1% of the patients) and in two dimensional size of tumor on computed tomography for 1 h. The resultant microspheres were collected by filtration through (93.6%). The cumulative survival rate was 54.3% at 1 year, 24.6% at 2 a membrane filter (3 //m pore diameter), rinsed 2 to 3 times (in 1 liter years, and 19.2% at 3 years, respectively, among 59 patients with of distilled water), and dried under reduced pressure for 5 days. -

Combining Paclitaxel and Lapatinib As Second-Line Treatment for Patients with Metastatic Transitional Cell Carcinoma: a Case Series
ANTICANCER RESEARCH 32: 3949-3952 (2012) Combining Paclitaxel and Lapatinib as Second-line Treatment for Patients with Metastatic Transitional Cell Carcinoma: A Case Series STÉPHANE CULINE, ZINEB SELLAM, LINDA BOUAITA, ELIAS ASSAF, CATHERINE DELBALDO, MURIEL VERLINDE-CARVALHO and DAMIEN POUESSEL Department of Medical Oncology, Henri Mondor Hospital, Créteil, France Abstract. Background: Current first-line cisplatin-based trial comparing vinflunine with best supportive care (BSC) combination chemotherapy regimens provide interesting to BSC alone, an estimated difference in overall survival response rates but limited impact on survival for patients with (OS) of 2 months was reached in the intent-to-treat metastatic transitional cell carcinoma of the urothelium. Such population. However, a significant difference in OS was only results leave a significant patient population in need of salvage seen after removing patients who had major protocol therapy. Patients and Methods: As the epidermal growth factor violations (2). Therefore therapy for patients who fail first- receptors 1 and 2 (EGFR and HER2) are frequently line cisplatin-based chemotherapy remains a highly unmet overexpressed in urothelial carcinoma, we explored the medical need. feasibility of a combination of paclitaxel (80 mg/m2/week) and In a phase II study led by the French Genito-Urinary lapatinib (1,500 mg orally daily) for six patients who were Tumor group (GETUG), the activity of weekly paclitaxel as treated after failure of first-line platinum-based chemotherapy. second-line chemotherapy was assessed in 45 patients with Results: Only one out of six patients was able to receive the MTCCU. A low objective response rate (9%) along with a full doses during the first six weeks of treatment, while grade high rate of stabilization (38%) suggested limited impact as 2 or 3 diarrhea events required lapatinib dose reduction (one a single agent (3). -

Phenotype Microarrays Panels PM-M1 to PM-M14
Phenotype MicroArrays™ Panels PM-M1 to PM-M14 for Phenotypic Characterization of Mammalian Cells Assays: Energy Metabolism Pathways Ion and Hormone Effects on Cells Sensitivity to Anti-Cancer Agents and for Optimizing Culture Conditions for Mammalian Cells PRODUCT DESCRIPTIONS AND INSTRUCTIONS FOR USE PM-M1 Cat. #13101 PM-M2 Cat. #13102 PM-M3 Cat. #13103 PM-M4 Cat. #13104 PM-M5 Cat. #13105 PM-M6 Cat. #13106 PM-M7 Cat. #13107 PM-M8 Cat. #13108 PM-M11 Cat. #13111 PM-M12 Cat. #13112 PM-M13 Cat. #13113 PM-M14 Cat. #13114 © 2016 Biolog, Inc. All rights reserved Printed in the United States of America 00P 134 Rev F February 2020 - 1 - CONTENTS I. Introduction ...................................................................................................... 2 a. Overview ................................................................................................... 2 b. Background ............................................................................................... 2 c. Uses ........................................................................................................... 2 d. Advantages ................................................................................................ 3 II. Product Description, PM-M1 to M4 ................................................................ 3 III. Protocols, PM-M1 to M4 ................................................................................. 7 a. Materials Required .................................................................................... 7 b. Determination -

Monotherapy with Pixantrone in Histologically Confirmed Relapsed Or
research paper Monotherapy with pixantrone in histologically confirmed relapsed or refractory aggressive B-cell non-Hodgkin lymphoma: post-hoc analyses from a phase III trial Ruth Pettengell,1 Catherine Sebban,2 This post hoc analysis of a phase 3 trial explored the effect of pixantrone in Pier Luigi Zinzani,3 Hans Gunter patients (50 pixantrone, 47 comparator) with relapsed or refractory aggres- 4 5 Derigs, Sergey Kravchenko, Jack W. sive B-cell non-Hodgkin lymphoma (NHL) confirmed by centralized histo- 6 7 Singer, Panteli Theocharous, Lixia logical review. Patients received 28-d cycles of 85 mg/m2 pixantrone 7 8 Wang, Mariya Pavlyuk, Kahina M. dimaleate (equivalent to 50 mg/m2 in the approved formulation) on days Makhloufi8 and Bertrand Coiffier9,10 1, 8 and 15, or comparator. The population was subdivided according to 1St George’s University of London, London, UK, 2 previous rituximab use and whether they received the study treatment as Leon Berard Cancer Centre, Lyon, France, – 3Institute of Haematology “Le A Seragnoli”, 3rd or 4th line. Median number of cycles was 4 (range, 2 6) with pix- – University of Bologna, Bologna, Italy, 4St€adt Kli- antrone and 3 (2 6) with comparator. In 3rd or 4th line, pixantrone was Á Á nikum, Frankfurt-Hoeschest, Klinik fur€ Innere associated with higher complete response (CR) (23 1% vs. 5 1% compara- Medizin III, Frankfurt am Main, Germany, tor, P = 0Á047) and overall response rate (ORR, 43Á6% vs. 12Á8%, 5Chemotherapy and Intensive Treatment of Hae- P = 0Á005). In 3rd or 4th line with previous rituximab (20 pixantrone, 18 matology Diseases, Haematology Scientific Centre comparator), pixantrone produced better ORR (45Á0% vs. -

Towards the Reduction of Occupational Exposure To
TOWARDS THE REDUCTION OF OCCUPATIONAL EXPOSURE TO CYTOTOXIC DRUGS by Cristian Vasile Barzan B.Sc. Simon Fraser University, 2007 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate Studies (Occupational and Environmental Hygiene) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2010 © Cristian Vasile Barzan, 2010 Abstract Background : One of the most powerful and widely used techniques in cancer treatment is the use of cytotoxic drugs in chemotherapy. These drugs are inherently hazardous with many of them causing carcinogenic, mutagenic or teratogenic health outcomes. Occupational exposure to cytotoxic drugs is of great concern due to their lack of selectivity between healthy and unhealthy cells. Widespread cytotoxic drug contamination has been reported in North America, Europe and Australia. Current cleaning protocols for hazardous antineoplastic drugs include the use of disinfectants and oxidizing agents, such as household bleach. Aim : The thesis project focused on two objectives: 1) hypothesize and confirm potential hazardous by- products arising from cleaning cyclophosphamide, a widely used cytotoxic drugs, with household bleach, a commonly used cleaning agent; 2) develop an effective and safe cleaning agent for cytotoxic drugs in order to prevent and eliminate exposure to these drugs. Methods : The gas chromatograph mass spectrum (GC/MS) was used to analyze the decomposition of cyclophosphamide by household bleach (5.25% hypochlorite). The reaction was conducted in a test-tube and the by-products extracted and derivatized prior to analysis. Multiple cleaning agent compositions were tested on 10x10cm stainless steel plates spiked with the two model cytotoxic drugs, cyclophosphamide and methotrexate. -
Transitional Cell Carcinoma: Options Beyond Nsaids Julie Marie Gillem, DVM, DACVIM (Oncology) Overview
Transitional Cell Carcinoma: Options Beyond NSAIDs Julie Marie Gillem, DVM, DACVIM (Oncology) Overview ✦ Background ✦ Surgical Options ✦ Pathology ✦ Medical Options ✦ Location and staging ✦ Radiation Therapy ✦ Behavior Options ✦ Etiology and risk factors ✦ Palliative care ✦ Work up and diagnosis ✦ What about cats? Objectives ✦ How do we determine when NSAIDs fail? ✦ When should we intervene with surgery, chemotherapy, radiation therapy, and additional palliative care? Pathology ✦ ~2% of canine cancer ✦ Invasive transitional cell carcinoma (TCC) most common ✦ Others: SCC, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, fibroma, and other mesenchymal tumors Location and Staging ✦ TCC in dogs most often found in the trigone of the bladder ✦ Series of 102 dogs at PUVTH ✦ Urethra and bladder in 56% ✦ Prostate involvement in 29% male dogs ✦ Lymph node mets in 16% at diagnosis ✦ Distant mets in 14% at diagnosis ✦ Distant mets in 50% at death Location ✦ TCC in dogs most often is found in the trigone region of the bladder. ✦ In a series of dogs with TCC examined at the PUVTH, the tumor involved the urethra as well as the bladder in 57 of 102 dogs (56%), and it involved the prostate in 11 of 38 (29%) male dogs. WHO Staging ✦ 78% T2 tumors ✦ 20% T3 tumors Biological Behavior ✦ At diagnosis: ✦ Regional lymph node metastasis in 12-46 % (Norris et al 1992, Knapp et al 2000, Blackburn et al 2013) ✦ Distant metastasis in 16- 23% (Norris et al 1992, Blackburn et al 2013) ✦ Distant metastasis in 50% at death (Norris et al 1992, Knapp et al -

Oxaliplatin, 5-Fluorouracil and Leucovorin (FOLFOX) As Second- Line Therapy for Patients with Advanced Urothelial Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 7, No. 36 Clinical Research Paper Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) as second- line therapy for patients with advanced urothelial cancer Sheng Zhang1, Hongxi Xue2, Qiang Chen3 1Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China 2Rizhao City Hospital of Traditional Chinese Medicine, Rizhao, China 3Department of Clinical Biochemistry, School of Public Health, Taishan Medical University, Tai’an, China Correspondence to: Sheng Zhang, email: [email protected] Keywords: urothelial cancer, oxaliplatin, leucovorin, 5-fluorouracil, clinical trial Received: February 08, 2016 Accepted: June 30, 2016 Published: July 07, 2016 ABSTRACT There is currently no standard treatment for metastatic urothelial cancer after failure of cisplatin-based therapy. The present retrospective study investigated the efficacy and safety of oxaliplatin plus 5-fluorouracil (5-FU) and leucovorin (LV) (FOLFOX) in locally advanced or metastatic urothelial cancer patients following cisplatin-based treatment. Thirty-three patients who had received one or two cisplatin-based regimens were treated with oxaliplatin (85 mg/m2) as a 2-h infusion on day 1, LV (200 mg/m2) as a 2-h infusion followed by bolus 5-FU (400 mg/m2) on day 1, or a 44-h continuous 5-FU (1,200 mg/m2) infusion. Patients were a mean of 67 years old with two involved organs. Metastases were mostly in the lung (43%), lymph nodes (51%) and liver (46%). Based on an intention-to-treat analysis, nine patients achieved a partial response, with an overall response rate of 27%. Eight (24%) patients had stable disease. -

Functional Similarity of Anticancer Drugs by MTT Bioassay
cer Scien an ce C & f o T l h a e Hiwasa et al., J Cancer Sci Ther 2011, 3.10 n r a Journal of r p u 1000099 y o J DOI: 10.4172/1948-5956. ISSN: 1948-5956 Cancer Science & Therapy Rapid Communication Open Access Functional Similarity of Anticancer Drugs by MTT Bioassay Takaki Hiwasa1*,Takanobu Utsumi1, Mari Yasuraoka1, Nana Hanamura1, Hideaki Shimada2, Hiroshi Nakajima3, Motoo Kitagawa4, Yasuo Iwadate4,5, Ken-ichiro Goto6, Atsushi Takeda7, Kenzo Ohtsuka8, Hiroyoshi Ariga9 and Masaki Takiguchi1 1Department of Biochemistry, and Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 2Department of Surgery, School of Medicine, Toho University, Ota-ku, Tokyo 143-8541, Japan 3Department of Molecular Genetics, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 4Department of Molecular and Tumor Pathology, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 5Department of Neurological Surgery, Chiba University, Graduate School of Medicine, Chuo-ku, Chiba 260-8670, Japan 6Department of Orthopaedic Surgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido, Chiba 284-0003, Japan 7Laboratory of Biochemistry, Graduate School of Nutritional Sciences, Sagami Women’s University, Sagamihara, Kanagawa 252-0383, Japan 8Laboratory of Cell & Stress Biology, Department of Environmental Biology, Chubu University, Matsumoto-cho, Kasugai, Aichi 487-8501, Japan 9Graduate School of Pharmaceutical Sciences, Hokkaido University, Kita-ku, Sapporo 060-0812, Japan Abstract We prepared normal or Ha-ras-transformed NIH3T3 cells transfected stably or transiently with various tumor- related genes. The chemosensitivity of the transfected clones to 16 anticancer drugs was compared to the parental control cells using the MTT assay. -

Safe Handling of Cytotoxic, Monoclonal Antibody & Hazardous Non-Cytotoxic Drugs
PROCEDURE SAFE HANDLING OF CYTOTOXIC, MONOCLONAL ANTIBODY & HAZARDOUS NON-CYTOTOXIC DRUGS TARGET AUDIENCE All nursing, pharmacy and medical staff involved with dispensing, preparation, or administration of medicines. STATE ANY RELATED PETER MAC POLICIES, PROCEDURES OR GUIDELINES Administration and Management of Anti-Cancer Drugs Administration of Cytotoxics in the Home/Community Collection and Disposal of Soiled Linen Dangerous Goods and Hazardous Substances Environmental Management Individual Personal Protective Equipment (Cancer Research Division) Management of Cytotoxic Drug Spill Medication Management Medication Management for Nurses Pharmaceutical Review & Medication Supply Personal Protective Equipment Administration of Intravesical Immunotherapy BCG PURPOSE This procedure provides direction to all hospital staff involved in the management, preparation, transportation, administration of hazardous drugs and related wastes. In particular, safe handling practices for cytotoxic and hazardous non-cytotoxic drugs are outlined. BACKGROUND Hazardous drugs are regulated medicines that have been classified by the National Institute for Occupational Safety and Health (NIOSH) of the United States and/or the Cancer Institute New South Wales as posing a risk to health from occupational exposure. Exposure to hazardous drugs can result in adverse health effects in healthcare workers. The health risk depends on how much exposure a worker has to these drugs and the specific toxicity of the drug. The occupational exposure risk of hazardous drugs is therefore evaluated according to risk of internalisation (by ingestion, absorption through mucous membranes, and penetration of skin) and risk of toxicity (carcinogenicity, genotoxicity, teratogenicity, and reproductive or fertility impairment, organ toxicity) at low doses and continuous exposure. Hazardous drugs include both cytotoxic and non-cytotoxic medicines such as chemotherapy, monoclonal antibodies, immunomodulatory drugs, and some anti-infective drugs. -

Metabolic Carbonyl Reduction of Anthracyclines — Role in Cardiotoxicity and Cancer Resistance
Invest New Drugs DOI 10.1007/s10637-017-0443-2 REVIEW Metabolic carbonyl reduction of anthracyclines — role in cardiotoxicity and cancer resistance. Reducing enzymes as putative targets for novel cardioprotective and chemosensitizing agents Kamil Piska1 & Paulina Koczurkiewicz1 & Adam Bucki 2 & Katarzyna Wójcik-Pszczoła1 & Marcin Kołaczkowski2 & Elżbieta Pękala1 Received: 23 November 2016 /Accepted: 17 February 2017 # The Author(s) 2017. This article is published with open access at Springerlink.com Summary Anthracycline antibiotics (ANT), such as doxoru- monoHER, curcumin, (−)-epigallocatechin gallate, resvera- bicin or daunorubicin, are a class of anticancer drugs that are trol, berberine or pixantrone, and their modulating effect on widely used in oncology. Although highly effective in cancer the activity of ANT is characterized and discussed as potential therapy, their usefulness is greatly limited by their mechanism of action for novel therapeutics in cancer cardiotoxicity. Possible mechanisms of ANT cardiotoxicity treatment. include their conversion to secondary alcohol metabolites (i.e. doxorubicinol, daunorubicinol) catalyzed by carbonyl re- Keywords Anthracyclines . Cardiotoxicity . Resistance . ductases (CBR) and aldo-keto reductases (AKR). These me- Pharmacokinetics . Drug metabolism . Anticancer agents tabolites are suspected to be more cardiotoxic than their parent compounds. Moreover, overexpression of ANT-reducing en- zymes (CBR and AKR) are found in many ANT-resistant Introduction cancers. The secondary metabolites show decreased cytotoxic properties and are more susceptible to ABC-mediated efflux Anthracyclines (ANT) are a class of cell-cycle non-specific than their parent compounds; thus, metabolite formation is anticancer antibiotics that were first isolated from the considered one of the mechanisms of cancer resistance. Streptomyces genus in the early 1960s. -
Guideline for the Management of Extravasation
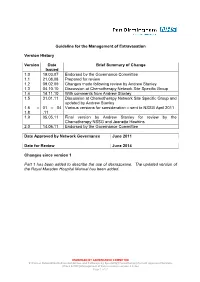
Guideline for the Management of Extravasation Version History Version Date Brief Summary of Change Issued 1.0 19.03.07 Endorsed by the Governance Committee 1.1 21.08.08 Prepared for review 1.2 09.02.09 Changes made following review by Andrew Stanley 1.3 04.10.10 Discussion at Chemotherapy Network Site Specific Group 1.4 14.11.10 With comments from Andrew Stanley 1.5 31.01.11 Discussion at Chemotherapy Network Site Specific Group and updated by Andrew Stanley 1.6 – 01 – 04 Various versions for consideration – sent to NSSG April 2011 1.8 .11 1.9 05.05.11 Final version by Andrew Stanley for review by the Chemotherapy NSSG and Jeanette Hawkins 2.0 14.06.11 Endorsed by the Governance Committee Date Approved by Network Governance June 2011 Date for Review June 2014 Changes since version 1 Part 1 has been added to describe the use of dexrazoxane. The updated version of the Royal Marsden Hospital Manual has been added. ENDORSED BY GOVERNANCE COMMITTEE S:\Cancer Network\Guidelines\Guidelines and Pathways by Speciality\Chemotherapy\Current Approved Versions (Word & PDF)\Management of Extravasation version 2.0.doc Page 1 of 21 1 Scope of the Guideline This guidance has been produced to support the following: The prevention of the extravasation of intravenous anti-cancer drugs. The early detection of the extravasation of intravenous anti-cancer drugs. The treatment of the extravasation of intravenous anti-cancer drugs. 2 Guideline Statement Statement 2 The Network Site Specific Group has agreed to adopt the Royal Marsden Hospital Manual of Clinical Nursing Procedures 7th Edition; Blackwell Publishing (2008), chapter on extravasation, with the addition of a section on dexrazoxane.