Ground Water Quality and Its Health Impact Analysis in an Industrial Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
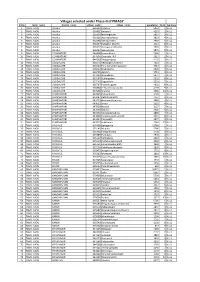
Villages Selected Under Phase-II of PMAGY
Villages selected under Phase-II of PMAGY Sl No State_name District_name village_code village_name population Rank Ispmagy 1 TAMIL NADU Ariyalur 636312 Kulumur 5052 1 NULL 2 TAMIL NADU Ariyalur 636451 Pottaveli 4261 2 NULL 3 TAMIL NADU Ariyalur 636319 Namangunam 3897 3 NULL 4 TAMIL NADU Ariyalur 636311 Ayanathathanur 3823 4 NULL 5 TAMIL NADU Ariyalur 636368 Vethiyarvettu 3503 5 NULL 6 TAMIL NADU Ariyalur 636357 Kattathur (North) 2113 6 NULL 7 TAMIL NADU Ariyalur 636372 Kattagaram (South) 2093 7 NULL 8 TAMIL NADU Ariyalur 636453 Sennivanam 1870 8 NULL 9 TAMIL NADU COIMBATORE 644408 Kanuvakkarai 2646 1 NULL 10 TAMIL NADU COIMBATORE 644876 Anamalai R.F. 2014 2 NULL 11 TAMIL NADU COIMBATORE 644530 Thoppampatti 1135 3 NULL 12 TAMIL NADU CUDDALORE 636574 Veerapperumanallur 7324 1 NULL 13 TAMIL NADU CUDDALORE 636544 Perumalnaickenpalayam 7317 2 NULL 14 TAMIL NADU CUDDALORE 636622 Madalpattu 6028 3 NULL 15 TAMIL NADU CUDDALORE 636705 Agaram 5715 4 NULL 16 TAMIL NADU CUDDALORE 637229 Sirupakkam 5477 5 NULL 17 TAMIL NADU CUDDALORE 637167 U.Mangalam 5292 6 NULL 18 TAMIL NADU CUDDALORE 637103 Seppakkam 5201 7 NULL 19 TAMIL NADU CUDDALORE 636707 Puvanikuppam 4992 8 NULL 20 TAMIL NADU CUDDALORE 636860 C.Thandeswaranallur 4159 9 NULL 21 TAMIL NADU CUDDALORE 637238 Orangur 3966 10 NULL 22 TAMIL NADU DHARMAPURI 643335 Palayampalli 4195 1 NULL 23 TAMIL NADU DHARMAPURI 643487 Pethathampatti 4137 2 NULL 24 TAMIL NADU DHARMAPURI 643223 Hanumanthapuram 3940 3 NULL 25 TAMIL NADU DHARMAPURI 643613 Sivadi 3532 4 NULL 26 TAMIL NADU DHARMAPURI 643329 Navalai 3127 -

Perambalur District
CENSUS OF INDIA 2011 TOTAL POPULATION AND POPULATION OF SCHEDULED CASTES AND SCHEDULED TRIBES FOR VILLAGE PANCHAYATS AND PANCHAYAT UNIONS PERAMBALUR DISTRICT DIRECTORATE OF CENSUS OPERATIONS TAMILNADU ABSTRACT PERAMBALUR DISTRICT No. of Total Total Sl. No. Panchayat Union Total Male Total SC SC Male SC Female Total ST ST Male ST Female Village Population Female 1 Perambalur 20 1,00,245 50,761 49,484 32,523 16,187 16,336 160 88 72 2 Veppanthattai 29 1,25,539 62,268 63,271 38,176 18,969 19,207 1,555 784 771 3 Alathur 39 1,05,986 52,660 53,326 28,928 14,259 14,669 209 105 104 4 Veppur 33 1,36,247 68,215 68,032 50,877 25,839 25,038 212 100 112 Grand Total 121 4,68,017 2,33,904 2,34,113 1,50,504 75,254 75,250 2,136 1,077 1,059 PERAMBALUR PANCHAYAT UNION Sl. Name of Population Scheduled Castes Scheduled Tribes No. Panchayat Total Males Females Total Males Females Total Males Females 1 2 3 4 5 6 7 8 9 10 11 1 Alambadi 11,757 6,096 5,661 3,562 1,774 1,788 11 7 4 2 Ammapalayam 5,692 2,879 2,813 1,835 917 918 46 20 26 3 Ayyalur 3,481 1,730 1,751 1,337 664 673 - - - 4 Bommanappady 3,167 1,557 1,610 1,579 759 820 - - - 5 Chathiramanai 3,543 1,738 1,805 1,088 525 563 2 2 - 6 Elambalur 10,357 5,606 4,751 2,503 1,262 1,241 11 6 5 7 Esanai 6,385 3,142 3,243 3,374 1,646 1,728 26 12 14 8 Kalarampatti 3,418 1,687 1,731 1,684 816 868 1 - 1 9 Kalpadi 6,482 3,197 3,285 1,721 865 856 1 1 - 10 Kavulpalayam 3,086 1,597 1,489 64 35 29 - - - 11 Keelakkarai 1,145 582 563 2 1 1 - - - 12 Koneripalayam 1,800 890 910 441 206 235 - - - 13 Ladapuram 8,893 4,473 4,420 3,757 1,900 1,857 33 19 14 14 Melapuliyur 5,686 2,983 2,703 2,503 1,322 1,181 21 18 3 15 Nochiyam 3,611 1,822 1,789 647 320 327 1 - 1 16 Pudhunaduvalur 2,416 1,233 1,183 744 374 370 - - - 17 Sengunam 4,621 2,319 2,302 2,125 1,071 1,054 - - - 18 Siruvachur 5,616 2,672 2,944 2,016 956 1,060 1 - 1 19 Vadakumadhavi 4,189 2,112 2,077 671 344 327 6 3 3 20 Velur 4,900 2,446 2,454 870 430 440 - - - TOTAL 1,00,245 50,761 49,484 32,523 16,187 16,336 160 88 72 VEPPANTHATTAI PANCHAYAT UNION Sl. -

ANSWERED ON:11.05.2005 AUTOMATIC and MODERN TELEPHONE EXCHANGES in TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy
GOVERNMENT OF INDIA COMMUNICATIONS AND INFORMATION TECHNOLOGY LOK SABHA UNSTARRED QUESTION NO:6879 ANSWERED ON:11.05.2005 AUTOMATIC AND MODERN TELEPHONE EXCHANGES IN TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy Will the Minister of COMMUNICATIONS AND INFORMATION TECHNOLOGY be pleased to state: (a) the details of automatic and modern telephone exchanges set up in Tamil Nadu during the last three years, location- wise; (b) the details of such exchanges proposed to be set up in Tamil Nadu during the current year; (c) the details of the telephone exchanges whose capacities were expanded in the current financial year; and (d) the details of telephone exchanges where waiting list for telephone connection still exists? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS ANDINFORMATION TECHNOLOGY (DR. SHAKEEL AHMAD) (a) The details of automatic and modern telephone exchanges set up in Tamilnadu during the last three years are given in the Annexures- I(a), I(b) & I(c). (b) The details of such exchanges proposed to be set up in Tamilnadu during the current year are given in Annexure-II. (c) The details of the telephone exchanges whose capacities were expanded in the current financial year are given at Annexure-III. (d) The details of telephone exchanges where waiting list for telephone connection still exists are given in Annexure- IV. ANNEXURE-I(a) DETAILS OF TELEPHONE EXCHANGES SET UP DURING 2002-03 IN TAMILNADU Sl Name of Exchange Capacity Type/Technology District No.(Location) 1 Avinashi-II 4000 CDOTMBMXL Coimbatore 2 K.P.Pudur -
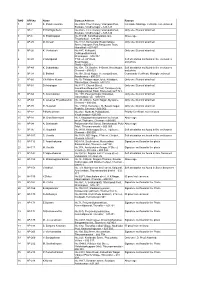
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Caste List of MBC and DC of Tamilnadu State
Cast List of Most Backward Classes 1. Ambalakarar 2. Andipandaram 3. Bestha, Siviar 4. Bhatraju( other than Kshatriya Raju ) 5. Boyar, Oddar 6. Dasari 7. Dommara 8. Eravallar( except in Kanniyakumari District and ShencottahTaluk of Tirunelveli District where the community is a Scheduled Tribe ) 9. Isaivellalar 10. Jambuvanodai 11. Jangam 12. Jogi 13. KonguChettiar( in Coimbatore and Erode Districts only ) 14. Koracha 15. Kulala (including Kuyavar and Kumbarar ) 16. KunnuvarMannadi 17. Kurumba 18. KuruhiniChetty 19. Maruthuvar, Navithar, Mangala, Velakattalavar, Velakatalanair and Pronopakari 20. MondGolla 21 MoundadanChetty 22. Mahendra, Medara 23. Mutlakampatti 24. Narikoravar 25. Nokkar 26. Vanniakula Kshatriya ( includingVanniyar, Vanniya, VanniaGounder, Gounder or Kander, Padayachi, Palli and AgnikulaKshatriya ) 27. Paravar( except in Kanniyakumari District and ShencottahTaluk of Tirunelveli District where the Community is a Scheduled Caste) (including converts to Christianity ) 28. Meenavar( Parvatharajakulam, Pattanavar, Sembadavar) ( including converts to Christianity ) 29. Mukkuvar or Mukayar( including converts to Christianity) 30. PunnanVettuvaGounder 31. Pannayar( other than Kathikarar in Kanniyakumari District) 32. SathathaSrivaishnava( includingSathani, Chattadi and Chattada Srivaishnava) 33. SozhiaChetty 34. TelugupattyChetty 35. ThottiaNaicker( includingRajakambalam, Gollavar, Sillavar, Thockalavar and ThozhuvaNaicker ) 36. Thondaman 37. Valaiyar( includingChettinadValayars ) 38. Vannar( SalavaiThozhilalar ) ( including -
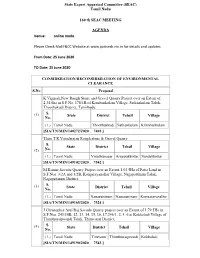
Tamil Nadu 160 Th SEAC MEETING
State Expert Appraisal Committee (SEAC) Tamil Nadu 160 th SEAC MEETING AGENDA Venue: online mode Please Check MoEF&CC Website at www.parivesh.nic.in for details and updates From Date: 25 June 2020 TO Date: 25 June 2020 CONSIDERATION/RECONSIDERATION OF ENVIRONMENTAL CLEARANCE S.No Proposal K.Vignesh,New Rough Stone and Gravel Quarry Project over an Extent of 2.34.0ha in S.F.No. 178/1B of Kombankulam Village, Sathankulam Taluk, Thoothukudi District, Tamilnadu. S. (1) State District Tehsil Village No. (1.) Tamil Nadu Thoothukkudi Sathankulam KOmbankulam [SIA/TN/MIN/140272/2020 , 7403 ] Thiru.T.R.Varadarajan Rough stone & Gravel Quarry S. State District Tehsil Village (2) No. (1.) Tamil Nadu Virudhunagar Aruppukkottai Sundakkottai [SIA/TN/MIN/149102/2020 , 7542 ] M.Kumar,Savudu Quarry Project over an Extent 1.03.5Ha of Patta Land in S.F.Nos. 3/2A and 3/2B, Kongarayanallur Village, Nagapattinam Taluk, Nagapattinam District. S. (3) State District Tehsil Village No. (1.) Tamil Nadu Nagapattinam Nagapattinam Kongarayanallur [SIA/TN/MIN/149165/2020 , 7524 ] J.Christopher Arul Raj,Savudu Quarry project over an Extent of 1.79.5Ha in S.F.Nos. 243/10B, 12, 13, 14, 15, 16, 17,246/1, 2, 4, 6 at Kokkaladi Village of Thiruthuraipoondi Taluk, Thiruvarur District, S. (4) State District Tehsil Village No. (1.) Tamil Nadu Tiruvarur Thiruthuraipoondi Kokkaladi [SIA/TN/MIN/149190/2020 , 7543 ] Tvl. City Blue Metal, New Rough Stone Quarry Project over an Extent of 1.01.0Ha of Government land in S.F.No. 2(Part – 2) of Ellappudayampatti Village, Harur Taluk, Dharmapuri District, Tamilnadu. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2013 [Price: Rs. 54.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 41] CHENNAI, WEDNESDAY, OCTOBER 23, 2013 Aippasi 6, Vijaya, Thiruvalluvar Aandu–2044 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 2893-3026 Notice .. 3026-3028 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 43888. My son, D. Ramkumar, born on 21st October 1997 43891. My son, S. Antony Thommai Anslam, born on (native district: Madurai), residing at No. 4/81C, Lakshmi 20th March 1999 (native district: Thoothukkudi), residing at Mill, West Colony, Kovilpatti, Thoothukkudi-628 502, shall Old No. 91/2, New No. 122, S.S. Manickapuram, Thoothukkudi henceforth be known as D. RAAMKUMAR. Town and Taluk, Thoothukkudi-628 001, shall henceforth be G. DHAMODARACHAMY. known as S. ANSLAM. Thoothukkudi, 7th October 2013. (Father.) M. v¯ð¡. Thoothukkudi, 7th October 2013. (Father.) 43889. I, S. Salma Banu, wife of Thiru S. Shahul Hameed, born on 13th September 1975 (native district: Mumbai), 43892. My son, G. Sanjay Somasundaram, born residing at No. 184/16, North Car Street, on 4th July 1997 (native district: Theni), residing Vickiramasingapuram, Tirunelveli-627 425, shall henceforth at No. 1/190-1, Vasu Nagar 1st Street, Bank be known as S SALMA. -

Trichy FOC Centres Phone Numbers Land Line Mobile 9445853
Name of the Region : Trichy Fuse Off Call Centres Name of the Circle : Trichy Phone Numbers FOC Centres Land Line Mobile For BSNL Users:1912 9445853479 - TRICHY For others:04311912 9445853480 Name of the Circle : Karur Phone Numbers FOC Centres Land Line Mobile KARUR 1912 94445854093 Name of the Circle : Pudukkottai Phone Numbers FOC Centres Land Line Mobile Pudukkottai 04322-221523 ----- Landline Numbers Name of the Circle : TRICHY Elecy. Distn. Circle/Metro Section Sub-Division Division Section Name Phone No Sub Division Phone No Division Phone No Name Name Thennur 0431-2794237 Thillainagar 0431-2791467 0431 - Woraiyur 0431 -2794237 THENNUR 2794237 Srinivasanagar 0431 -2794237 Con-II/Rockfort 0431-2793220 Con-I/Urban/Trichy 0431-2793220 Rockfort 0431-2793220 0431- Cinthamani 0431 -2793220 ROCKFORT 0431 - 2793220 Maingauardgate 0431 -2793220 URBAN 2793131 110KV/K.Pettai 0431 -2706443 Palakkarai 0431-2793220 Gandhimarket 0431-2793220 Senthaneerpuram 0431 -2793220 0431 - PALAKKARAI Junction 0431 -2414749 2793220 Ponnagar 0431-2481858 Mahalakshminagar 0431 -2202525 Cantonment 0431-2460148 Mannarpuram 0431-2420145 Subramaniapuram 0431 -2420145 Up graded Code No: Sembattu 0431 -2341924 section 0431 Crawford 0431 -2471880 KK Nagar 0431 -2341032 Rural/ Trichy 0431-2422301 EAST 0431 Manikandam 0431-2680300 /TRICHY 242223 Tiruparaithurai 0431-2614322 RURAL / 0431- TRICHY 2422301 Somarasampettai 0431-2607271 110 KV SS/ Ammapettai 0431-2680300 110 KV SS/Alundur 0431-2680514 Tiruverumbur 0431-2512773 THIRUVERUMB 0431- Navalpattu 0431-2512224 UR -

Community List
ANNEXURE - III LIST OF COMMUNITIES I. SCHEDULED TRIB ES II. SCHEDULED CASTES Code Code No. No. 1 Adiyan 2 Adi Dravida 2 Aranadan 3 Adi Karnataka 3 Eravallan 4 Ajila 4 Irular 6 Ayyanavar (in Kanyakumari District and 5 Kadar Shenkottah Taluk of Tirunelveli District) 6 Kammara (excluding Kanyakumari District and 7 Baira Shenkottah Taluk of Tirunelveli District) 8 Bakuda 7 Kanikaran, Kanikkar (in Kanyakumari District 9 Bandi and Shenkottah Taluk of Tirunelveli District) 10 Bellara 8 Kaniyan, Kanyan 11 Bharatar (in Kanyakumari District and Shenkottah 9 Kattunayakan Taluk of Tirunelveli District) 10 Kochu Velan 13 Chalavadi 11 Konda Kapus 14 Chamar, Muchi 12 Kondareddis 15 Chandala 13 Koraga 16 Cheruman 14 Kota (excluding Kanyakumari District and 17 Devendrakulathan Shenkottah Taluk of Tirunelveli District) 18 Dom, Dombara, Paidi, Pano 15 Kudiya, Melakudi 19 Domban 16 Kurichchan 20 Godagali 17 Kurumbas (in the Nilgiris District) 21 Godda 18 Kurumans 22 Gosangi 19 Maha Malasar 23 Holeya 20 Malai Arayan 24 Jaggali 21 Malai Pandaram 25 Jambuvulu 22 Malai Vedan 26 Kadaiyan 23 Malakkuravan 27 Kakkalan (in Kanyakumari District and Shenkottah 24 Malasar Taluk of Tirunelveli District) 25 Malayali (in Dharmapuri, North Arcot, 28 Kalladi Pudukkottai, Salem, South Arcot and 29 Kanakkan, Padanna (in the Nilgiris District) Tiruchirapalli Districts) 30 Karimpalan 26 Malayakandi 31 Kavara (in Kanyakumari District and Shenkottah 27 Mannan Taluk of Tirunelveli District) 28 Mudugar, Muduvan 32 Koliyan 29 Muthuvan 33 Koosa 30 Pallayan 34 Kootan, Koodan (in Kanyakumari District and 31 Palliyan Shenkottah Taluk of Tirunelveli District) 32 Palliyar 35 Kudumban 33 Paniyan 36 Kuravan, Sidhanar 34 Sholaga 39 Maila 35 Toda (excluding Kanyakumari District and 40 Mala Shenkottah Taluk of Tirunelveli District) 41 Mannan (in Kanyakumari District and Shenkottah 36 Uraly Taluk of Tirunelveli District) 42 Mavilan 43 Moger 44 Mundala 45 Nalakeyava Code III (A). -

Linkages -3.7.2
3.7.2 Number of linkages with institutions/industries for internship, on-the-job training, project work, sharing of research facilities etc. during the 2014-20 Name of the partnering institution/ industry /research lab with Duration (From- S. No Title of the linkage Year of commencement Nature of linkage Name of the participant Link to document contact details to) Ernst&young LLP 07 January 437, Manapakkam, Chennai, 1 Internship 2018 to 2019 2019 to Student Internship Mr. N. Krishna Sagar http://bit.ly/2TQ3tEX Tamil Nadu 600125 05 April 2019 Phone: 044 6654 8100 Peritus solutions private limited/No.2, 1st Floor, Third Street, Sri 02 January 2 Internship Sakthi Vijaylakshmi Nagar, Off 100 Feet Bypass Road, Velachery 2018 to 2019 2019 to Student Internship Mr.MOHAMMED ZIYYAD A http://bit.ly/3ayUNZr - Chennai - 600 042, Tamil Nadu, Phone: +91 44 48608788 02 April 2019 National Payments Corporation of India 1001A, B wing, 10 Floor, 04 June 2018 3 Summer Internship The Capital, Bandra-Kurla Complex, Bandra (East), Mumbai - 400 2018 to 2019 to Student Internship C.Pooja Priyadarshini http://bit.ly/2vhcM6E 051 Phone - 022 4000 9100 04 August 2018 SIDSYNC Technologies Pvt Ltd/Spaces.Express Avenue EA 24 January Chambers tower II, No. 49/50L,, Whites Road, Royapettah, 4 Internship 2018 to 2019 2019 to Student Internship Mr.JOSHUA J http://bit.ly/2TPUDqI Chennai, Tamil Nadu 600002 24 April 2019 Phone: 098948 19871 TAP Turbo Engineers Private Limited, Ambattur, 20 Jan 2019 5 Internship Chennai 600 58 2018 to 2019 to Student Internship Ms. Sai Gayathri Mahajan http://bit.ly/2uollMu Contact: 0442625 7234 20 March 2019 Trail Cloud Innovation Services Pvt Ltd, 187, Square Space 19 Nov 2018 Business Center, 188, Thiruvalluvar Rd, Block 10, Panneer Mr. -

Chengalpattu District
DISTRICT DISASTER MANAGEMENT PLAN 2020 CHENGALPATTU DISTRICT District Disaster Management Authority Chengalpattu District, Tamil Nadu DISTRICT DISASTER MANAGEMENT PLAN 2020 DISTRICT DISASTER MANAGEMENT AUTHORITY CHENGALPATTU DISTRICT TAMIL NADU PREFACE Endowed with all the graces of nature’s beauty and abundance, the newly created district of Chengalpattu is a vibrant administrative entity on the North eastern part of the state of Tamil Nadu. In spite of the district’s top-notch status in terms of high educational, human development index and humungous industrial productivity, given its geography, climate and certain other socio-political attributes, the district administration and its people have to co-exist with the probabilities of hazards like floods, cyclone, Tsunami, drought, heat wave, lightning and chemical, biological, radiological and nuclear emergencies. The Disastrous events in the recent past like the Tsunami of 2004, the catastrophic floods of year 2015, the cyclone of year 2016 and most recently the COVID-19 pandemic, will serve as a testament to the district’s vulnerability to such hazards. How the society responds to such vagaries of nature decides the magnitude and intensity of the destruction that may entail hazardous events. It is against this back drop, the roll of the District Disaster Management Authority can be ideally understood. The change in perspective from a relief- based approach to a more holistic disaster management approach has already begun to gain currency among the policy makers due to its substantial success in efficient handling of recent disasters across the globe. The need of the hour, therefore, is a comprehensive disaster management plan which is participative and people-friendly with the component of inter- departmental co-ordination at its crux. -

A Detailed Property Analysis Report of RRP Akshaya in Maraimalai Nagar
PROPINSIGHT A Detailed Property Analysis Report 40,000+ 10,000+ 1,200+ Projects Builders Localities Report Created On - 7 Oct, 2015 Price Insight This section aims to show the detailed price of a project and split it into its various components including hidden ones. Various price trends are also shown in this section. Project Insight This section compares your project with similar projects in the locality on construction parameters like livability rating, safety rating, launch date, etc. What is Builder Insight PROPINSIGHT? This section delves into the details about the builder and tries to give the user a perspective about the history of the builder as well as his current endeavours. Locality Info This section aims to showcase various amenities viz. pre-schools, schools, parks, restaurants, hospitals and shopping complexes near a project. RRP Akshaya Maraimalai Nagar, Chennai 19.3 Lacs onwards Livability Score 6.2/ 10 Project Size Configurations Possession Starts 3 Towers 2,3 Bedroom Apartment Dec `16 Pricing Comparison Comparison of detailed prices with various other similar projects Pricing Trends Price appreciation and trends for the project as well as the locality What is PRICE INSIGHT? Price versus Time to completion An understanding of how the current project’s prices are performing vis-a-vis other projects in the same locality Demand Comparison An understanding of how the strong/weak is the demand of current project and the current locality vis-a-vis others Price Trend Of RRP Akshaya RRP Akshaya VS Maraimalai Nagar, Chennai