G:\Judgedr\MDL\MDL 2226-Darvon-03-05-12-Generic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bio/Pharmaceutical Outsourcing Report: November 2016
Volume 21, Number 11, November 2016 Bio/Pharmaceutical Outsourcing Report Part of PharmSource STRATEGIC ADVANTAGE Business Conditions 1 Business Conditions 1 PE-Owned Companies in Line to Change Hands PE-Owned Companies in Line to Change Hands 2 Commercial Dose Manufacturing and Mergers and acquisitions are a hot topic in the contract services industry these Packaging days, and there is a lot of curiosity about which companies are likely to change 2 New Manufacturing Deal, Temporary hands. Of course, any company is a target at any time, especially when the price is Closures and Recall for Patheon right, but insight to likely acquisition candidates can be gained by looking at the list 3 Commercial Dose Manufacturing and Packaging in Brief 3 Clinical Dose Manufacturing and leastof companies four years owned ago are by likelyprivate targets equity in (PE) the nextfirms. year On oraverage, two. PE firms tend to hold Packaging onto their investments for about five years, so companies that were acquired at 3 Marken to be Acquired by UPS The table on page 2 lists 23 CMC services companies that are PE-owned; the list 5 Clinical Dose Manufacturing and was derived from the PharmSource Strategic Advantage database “Search by Packaging in Brief Ownership” feature. It is sorted by the year each company was acquired, and 6 Side Effects: Impacts of Key Events on suggests that at least 13 CMOs and CDMOs are ripe for a transaction in the foresee- CMOs and CROs able future. 7 API — Large Molecule The sale of one company, Marken (Durham, N.C., USA), was announced just this 7 Samsung BioLogics Completes IPO month (see news item, Page 3); another, Capsugel (Morristown, N.J., USA), has been 8 API — Large Molecule in Brief mentioned in the business press as being prepared for an ownership change. -

Comparison of Oral Contraceptives and Non-Oral Alternatives
PL Detail-Document #290305 −This PL Detail-Document gives subscribers additional insight related to the Recommendations published in− PHARMACIST’S LETTER / PRESCRIBER’S LETTER March 2013 Comparison of Oral Contraceptives and Non-Oral Alternatives —More information about the use of contraceptives is available in our PL Detail-Document, Hormonal Contraception— Productsa Manufacturerb Estrogen Progestin LOW-DOSE MONOPHASIC PILLS Aviane-28 Teva EE 20 mcg Levonorgestrel 0.1 mg Falmina Novast Lessina Teva Lutera Actavis Orsythia Qualitest Sronyx Actavis Gildess Fe 1/20 Qualitest EE 20 mcg Norethindrone acetate Junel 1/20 Teva 1 mg Junel Fe 1/20 Teva Loestrin-21 1/20 Warner Chilcott/Teva Loestrin Fe 1/20 Warner Chilcott/Teva Microgestin 1/20 Actavis Microgestin Fe 1/20 Actavis Generess Fe chewable Actavis EE 25 mcg Norethindrone 0.8 mg Altavera Sandoz EE 30 mcg Levonorgestrel 0.15 mg Kurvelo Lupin Levora Actavis Marlissa Glenmark Nordette-28 Duramed/Teva Portia-28 Teva Cryselle-28 Teva EE 30 mcg Norgestrel 0.3 mg Elinest Novast Low-Ogestrel-21 Actavis Low-Ogestrel-28 Actavis Lo/Ovral-28 Wyeth Gildess Fe 1.5/30 Qualitest EE 30 mcg Norethindrone acetate Junel 1.5/30 Teva 1.5 mg Junel Fe 1.5/30 Teva Loestrin 1.5/30-21 Warner Chilcott/Teva Loestrin Fe 1.5/30 Warner Chilcott/Teva Microgestin 1.5/30 Actavis Microgestin Fe 1.5/30 Actavis More. Copyright © 2013 by Therapeutic Research Center 3120 W. March Lane, Stockton, CA 95219 ~ Phone: 209-472-2240 ~ Fax: 209-472-2249 www.PharmacistsLetter.com ~ www.PrescribersLetter.com ~ www.PharmacyTechniciansLetter.com -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -
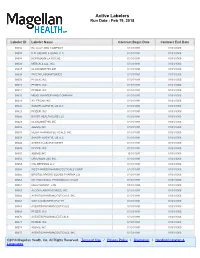
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC. -

Manufacturers/Wholesalers Street City ST Zip 454 Life Sciences Branford CT A.L.I Holdings, LLC A.L.I
Manufacturers/Wholesalers Street City ST Zip 454 Life Sciences Branford CT A.L.I Holdings, LLC A.L.I. Imaging Systems Corp A.L.I. Technologies (International) LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Laboratories 100 Abbott Park Rd. Bldg. AP6D-1 Abbott Park IL 60064 Abbott Molecular Division Abbott Nutrition Products Division Abbott Pharmaceutical Products Group Abbott Point of Care Division Abbott Vascular Division Abraxis Bioscience, LLC. 11755 Wilshire Blvd., Suite 2000 Los Angeles CA 90025 Access Diabetic Supply, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Acorda Therapeutics, Inc. 15 Skyline Drive Hawthorne NY 10532 Advanced Neuromodulation Systems, Inc. Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Aesculap Implant Systems, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Alaven Pharmaceuticals, LLC 2260 Northwest parkway Suite A Marietta GA 30067 Alcon Laboratories, Inc. 6201 South Freeway Fort Worth TX 76134 Alexion Pharmaceuticals, Inc. 352 Knotter Drive Cheshire CT 06410 Alkermes, Inc. 88 Sidney Street Cambridge MA 02139 Allen Medical Systems, Inc. Allergan Sales, LLC Allergan USA, Inc. Allergan, Inc. 2525 Dupont Drive Irvine CA 92612 Alpharma Pharmaceuticals, Inc. Alpharma Pharmaceuticals, LLC AMAG Pharmaceuticals, Inc. 100 Hayden Avenue Lexington MA 02421 AMATECH Corporation American Medical Distributors, Inc. 100 New Highway N Amityville NY 11701 American Medical System, -

United States District Court for the Western District of Kentucky Louisville Division
Case 3:18-cv-00558-CRS Document 1 Filed 08/17/18 Page 1 of 299 PageID #: 1 UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF KENTUCKY LOUISVILLE DIVISION BAPTIST HEALTHCARE SYSTEM, INC. d/b/a BAPTIST HEALTH CORBIN, BAPTIST HEALTH LA GRANGE, BAPTIST HEALTH LEXINGTON, COMPLAINT BAPTIST HEALTH LOUISVILLE, BAPTIST HEALTH PADUCAH, and CASE NO. _______________________3:18-cv-558-CHB BAPTIST HEALTH FLOYD; and JURY TRIAL DEMANDED BAPTIST HEALTH MADISONVILLE, INC. d/b/a BAPTIST HEALTH MADISONVILLE; This Action Relates to: Case No. 1:17-MD-2804 and Hon. Dan A. Polster BAPTIST HEALTH RICHMOND, INC. d/b/a BAPTIST HEALTH RICHMOND; Under: and Organized Crime Control Act of 1970, IX, BOWLING GREEN WARREN COUNTY Racketeer Influenced and Corrupt COMMUNITY HOSPITAL Organizations Act, P.L. No. 91-452, 84 CORPORATION d/b/a THE MEDICAL State. 922 (1970), (codified at 18 U.S.C. §§ CENTER AT BOWLING GREEN, THE 1961-1967 (2012) (“RICO”) MEDICAL CENTER AT CAVERNA, AND THE MEDICAL CENTER AT Breach of Implied Warranty of Fitness for SCOTTSVILLE; a Particular Purpose, KRS 355.2-315 and Kentucky Consumer Protection Act, KRS 367.170 THE MEDICAL CENTER AT CLINTON Negligence COUNTY, INC. d/b/a THE MEDICAL CENTER AT ALBANY Wanton Negligence and Negligence Per Se THE MEDICAL CENTER AT Negligent Marketing FRANKLIN, INC. d/b/a THE MEDICAL CENTER AT FRANKLIN Negligent Distribution Plaintiffs, Nuisance v. Unjust Enrichment AMERISOURCEBERGEN DRUG CORPORATION; Case 3:18-cv-00558-CRS Document 1 Filed 08/17/18 Page 2 of 299 PageID #: 2 CARDINAL HEALTH, INC.; MCKESSON CORPORATION; PURDUE PHARMA L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; TEVA PHARMACEUTICAL INDUSTRIES, LTD.; TEVA PHARMACEUTICALS USA, INC.; CEPHALON, INC.; JOHNSON & JOHNSON; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC. -

2020 Annual Report 2020 PARTNER of the YEAR 3 Annual Comparison SUSTAINED, CONSISTENT GROWTH
Greater Together Annual Report 2020 Greater Together The emergence of the COVID-19 pandemic led to unprecedented challenges for the global healthcare industry in 2020, and West played a vital role in the race to deliver an effective vaccine for the virus. CUSTOMERS: OUR PARTNERS Through these challenging times, vaccine developers have trusted West as a partner of choice to help protect their sensitive biomolecules. This is a testament to West’s almost 100 years of experience and reputation for leadership in quality. We have met these high expectations through our ability to flex our operations within our global network to meet supply needs. These customers were seeking components to support the development work, clinical trials, and potentially post-approval launches, and we have From the start of this global been committed to scaling production to meet their future volume requests. pandemic, we have had two In addition, West is also supplying primary packaging components for therapeutics used to treat COVID-19, as well as critical components that are priorities: first, ensuring the included in some of the diagnostic kit products that are being used to detect health and safety of our team COVID-19. members; and second, making We are committed to doing our part to provide for the large-scale manufacture of high-quality components required to serve customer needs for the delivery sure our customers have a reliable of a safe, effective COVID-19 vaccine. This has resulted in growing our global supply of the components that workforce to approximately 9,200 team members, expanding facility and equipment resources and shifting to 24/7 operating schedules at several sites are critical to the containment to address the demand for components associated with COVID-19. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 55513 AMGEN USA 00074 ABBOTT LABORATORIES 58406 AMGEN/IMMUNEX 68817 ABRAXIS BIOSCIENCE, LLC 53746 AMNEAL PHARMACEUTICALS 16729 ACCORD HEALTHCARE INCORPORATED 65162 AMNEAL PHARMACEUTICALS LLC 42192 ACELLA PHARMACEUTICALS, LLC 69238 AMNEAL PHARMACEUTICALS, LLC 10144 ACORDA THERAPEUTICS, INC. 53150 AMNEAL-AGILA, LLC 00472 ACTAVIS 00548 AMPHASTAR PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 69918 AMRING PHARMACEUTICALS, INC. 45963 ACTAVIS INC. 66780 AMYLIN PHARMACEUTICALS, INC. 46987 ACTAVIS KADIAN LLC 55724 ANACOR PHARMACEUTICALS 49687 ACTAVIS KADIAN LLC 10370 ANCHEN PHARMACEUTICALS, INC. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 43595 ANGELINI PHARMA, INC. 61874 ACTAVIS PHARMA, INC. 62559 ANIP ACQUISITION COMPANY 67767 ACTAVIS SOUTH ATLANTIC 54436 ANTARES PHARMA, INC. 66215 ACTELION PHARMACEUTICALS U.S., INC. 52609 APO-PHARMA USA, INC. 52244 ACTIENT PHARMACEUTICALS 60505 APOTEX CORP. 75989 ACTON PHARMACEUTICALS 63323 APP PHARMACEUTICALS, LLC. 69547 ADAPT PHARMA INC. 43485 APRECIA PHARMACEUTICALS COMPANY 76431 AEGERION PHARMACEUTICALS, INC. 42865 APTALIS PHARMA US, INC 50102 AFAXYS, INC. 58914 APTALIS PHARMA US, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 13310 AR SCIENTIFIC, INC. 27241 AJANTA PHARMA LIMITED 08221 ARBOR PHARM IRELAND LIMITED 17478 AKORN INC 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 24090 AKRIMAX PHARMACEUTICALS LLC 24338 ARBOR PHARMACEUTICALS, INC. 68220 ALAVEN PHARMACEUTICAL, LLC 59923 AREVA PHARMACEUTICALS 00065 ALCON LABORATORIES, INC. 76189 ARIAD PHARMACEUTICALS, INC. 00998 ALCON LABORATORIES, INC. 24486 ARISTOS PHARMACEUTICALS, INC. -

Strayhorn V. Wyeth Pharmaceuticals Inc
Case 2:11-cv-02058-STA-cgc Document 112 Filed 08/08/12 Page 1 of 22 PageID 2694 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF TENNESSEE WESTERN DIVISION ______________________________________________________________________________ GLORIA STRAYHORN and JEREMY ) STRAYHORN, ) ) Plaintiffs, ) ) v. ) No. 11-2058-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; ) SCHWARZ PHARMA AG; UCB GmbH; ) ALAVEN PHARMACEUTICALS LLC; ) and ACTAVIS ELIZABETH LLC, ) ) Defendants. ) ______________________________________________________________________________ SARAH SPEED, ) ) Plaintiff, ) ) v. ) No. 11-2095-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; and ) WATSON LABORATORIES, INC., ) ) Defendants. ) ______________________________________________________________________________ KATHLEEN SIMMONS, ) ) Plaintiff, ) ) v. ) No. 11-2083-STA-cgc ) WYETH PHARMACEUTICALS, INC.; ) 1 Case 2:11-cv-02058-STA-cgc Document 112 Filed 08/08/12 Page 2 of 22 PageID 2695 WYETH LLC; WYETH, INC.; PFIZER, ) INC.; SCHWARZ PHARMA, INC.; ) ALAVEN PHARMACEUTICAL, LLC; ) and WATSON LABORATORIES, INC., ) ) Defendants. ) ______________________________________________________________________________ GORDON and JUDITH WEAVER; ) SHENA JOHNSON; DEAN BROWN; ) GWENDOLYN RUFF; EMMA ) KETRON; LARRY HUDSON; ANNA ) ODOM; MARILYN MONCIER; ) THELMA DONALD; NETTER ) GRIGGS; ORVIELL RHODES; SELMA ) CARTER; and GERTIE KING, ) ) Plaintiffs, ) ) v. ) No. 11-2134-STA-cgc ) -

Brief in Opposition to Certiorari ______
No. 19–1451 In the Supreme Court of the United States __________ SANOFI-AVENTIS DEUTSCHLAND, GMBH, PETITIONER v. MYLAN PHARMACEUTICALS INC., RESPONDENT __________ ON PETITION FOR A WRIT OF CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT __________ BRIEF IN OPPOSITION TO CERTIORARI __________ DOUGLAS H. CARSTEN STEFFEN N. JOHNSON Wilson Sonsini Counsel of Record Goodrich & Rosati, P.C. RICHARD TORCZON 12235 El Camino Real ADAM W. BURROWBRIDGE San Diego, CA 92130 Wilson Sonsini (858) 350–2300 Goodrich & Rosati, P.C. 1700 K Street, N.W. Washington, DC 20006 (202) 973–8800 [email protected] Counsel for Respondent QUESTIONS PRESENTED 1. Whether the Federal Circuit correctly conclud- ed that Sanofi, by failing to plead or otherwise raise a nonjurisdictional Appointments Clause challenge that was pleaded and briefed by dozens of other par- ties and was the subject of extensive public commen- tary, in any brief or at any time until a letter filed two months after oral argument in that court, forfeit- ed such a challenge to the underlying Patent Trial & Appeal Board ruling at issue. 2. Whether the Federal Circuit correctly affirmed the Patent Trial and Appeal Board’s fact-bound con- clusion that Sanofi’s patents were obvious because the purported invention—adding a nonionic surfac- tant to impede insulin glargine molecules from ag- gregating during storage—simply applied a known solution to a known insulin problem. ii RELATED PROCEEDINGS The following proceedings are directly related to the case: Mylan Pharms. Inc. v. Sanofi-Aventis Deutschland GMBH, IPR2017-01526 (P.T.A.B.), final written decision entered December 12, 2018. -

Lou Schmukler of BMS on Creating Supply Chain Excellence
The Official Magazine of ISPE September-October 2016 | Volume 36, Number 5 Lou Schmukler of BMS on Creating Supply Chain Excellence How to Fight a Bully: An Interview with Nicole Pierson Meet Your 2016-2017 Board of Directors Quarterly Report: BIOTECHNOLOGY www.PharmaceuticalEngineering.org Pharmaceutical Engineering | September-October 2016 | 1 WHY WE DO IT CLEAN AIR MATTERS camfilapc.com/cleanairmatters Farr Gold Series® Camtain Dust Collector Quad Pulse Package Dust Collector Dust, Mist and Fume Collectors AIR POLLUTION CONTROL www.camfilapc.com • e-mail: [email protected] • 855-405-2271 CHANGE IS OPPORTUNITY Some see change as a problem; we see change as an opportunity. Adapting to the evolving trends and ever- changing regulations in the life sciences industry is what we’re known for. We’re driven to find the right solution to the most technically challenging problems. And we’re satisfied only when we’ve produced results that make you successful. crbusa.com THE RELENTLESS PURSUIT OF SUCCESS. YOURS .™ Biological Animal Health OSD Blood Fractionation Fill/Finish Oligonucleo/Peptides Vaccines Medical Devices API’s Nutraceuticals Engineering | Architecture | Construction | Consulting Editor’s Voice A First Time for Everything My introduction to the world of biopharma occurred in June 2015 as I listened to Andy Skibo, then Chair of ISPE’s Board, deliver his “Biologics Supply Chain Risks” keynote address at the ISPE/FDA/PQRI Editorial Board Quality Manufacturing Conference in Washington, DC. He emphasized the potential effect of supply Editor in chief: Anna Maria di Giorgio chain risk on biologics, which are projected to account for 80% of the pipeline and 70% of sales revenue Managing editor: Amy Loerch by 2020—just three short years away.