Ph.D. Thesis Viktor Ulicsni
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -
Zoology SYLLABUS 2016
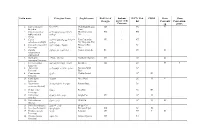
ST. PHILOMENA’S COLLEGE (AUTONOMOUS), MYSORE-570 015 Subject- ZOOLOGY Syllabus for B.A, Course under Semester Scheme. The Scheme of Teaching & Examination FROM THE ACADEMIC YEAR- 2016 Onwards Semester Title of the Paper Q. P. Code Teaching Scheme Examination Scheme Number Hours per Week Theory/ I A Total Practical Max Marks Proper Max. Marks hours Marks Theory Credits Practical Duration in in Duration MA 630 MA 631 IA T-60 T IA-10 3 I Animal Diversity I MA 632 PR-1 3 4.5 6 P-20 P IA-10 100 MA 633 PR- IA MB 630 MB 631 IA T-60 T IA-10 3 II Animal Diversity II MB632 PR-1 3 4.5 6 P-20 P IA-10 100 MB 633 PR- IA MC 630 T-60 T IA-10 MC 631 IA 3 III Animal Diversity III 3 4.5 6 P-20 P IA-10 100 MC 632 PR-1 MC 633 PR-1 MD 630 T-60 T IA-10 Biochemistry and MD 631 IA 3 IV 3 4.5 6 P-20 P IA-10 100 physiology MD 632 PR-1 MD 633 PR-1 ME 630 T IA T IA -20 Cell biology & ME 631 T IA 3 T- 80 3 4.5 6 P IA- 150 Immunology (P-5) ME 634 PR P- 40 10 ME 635 PR IA V ME 632 T IA Development biology T IA -20 ME 633 T IA 3 T- 80 & endocrinology 3 4.5 6 P IA- 150 ME 636 PR P- 40 (P-6) 10 ME 637 PR IA MF 630 T IA T IA -20 Genetics & evolution MF 631 T IA 3 T- 80 3 4.5 6 P IA- 150 (P-7) MF 634 PR P- 40 10 MF 635 PR IA VI MF 632 T IA Environmental T IA -20 MF 633 T IA 3 T- 80 Biology & Ethnology 3 4.5 6 P IA- 150 MF 636 PR P- 40 (P-8) 10 MF 637 PR IA ST. -

Changing Pattern of Spatio-Social Interrelationship of Hunting Community in Upper Dibang Valley
Changing Pattern of Spatio-Social Interrelationship of Hunting Community in Upper Dibang Valley, Arunachal Pradesh A Dissertation submitted To Sikkim University In Partial Fulfilment of the Requirements for the Degree of Master of Philosophy By MOHAN SHARMA Department of Geography School of Human Sciences February 2020 Date: 07/02/2020 DECLARATION I, Mohan Sharma, hereby declare that the research work embodied in the Dissertation titled “Changing Pattern of Spatio-Social Interrelationship of Hunting Community in Upper Dibang Valley, Arunachal Pradesh” submitted to Sikkim University for the award of the Degree of Master of Philosophy, is my original work. The thesis has not been submitted for any other degree of this University or any other University. (Mohan Sharma) Roll Number: 18MPGP01 Regd. No.: 18MPhil/GOG/01 Name of the Department: Geography Name of the School: Human Sciences Date: 07/02/2020 CERTIFICATE This is to certify that the dissertation titled “Changing Pattern of Spatio-Social Interrelationship of Hunting Community in Upper Dibang Valley, Arunachal Pradesh” submitted to Sikkim University for the partial fulfilment of the degree of Master of Philosophy in the Department of Geography, embodies the result of bonafide research work carried out by Mr. Mohan Sharma under our guidance and supervision. No part of the dissertation has been submitted for any other degree, diploma, associateship and fellowship. All the assistance and help received during the course of the investigation have been duly acknowledged by him. We recommend -

Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

Dipterists Forum
BULLETIN OF THE Dipterists Forum Bulletin No. 76 Autumn 2013 Affiliated to the British Entomological and Natural History Society Bulletin No. 76 Autumn 2013 ISSN 1358-5029 Editorial panel Bulletin Editor Darwyn Sumner Assistant Editor Judy Webb Dipterists Forum Officers Chairman Martin Drake Vice Chairman Stuart Ball Secretary John Kramer Meetings Treasurer Howard Bentley Please use the Booking Form included in this Bulletin or downloaded from our Membership Sec. John Showers website Field Meetings Sec. Roger Morris Field Meetings Indoor Meetings Sec. Duncan Sivell Roger Morris 7 Vine Street, Stamford, Lincolnshire PE9 1QE Publicity Officer Erica McAlister [email protected] Conservation Officer Rob Wolton Workshops & Indoor Meetings Organiser Duncan Sivell Ordinary Members Natural History Museum, Cromwell Road, London, SW7 5BD [email protected] Chris Spilling, Malcolm Smart, Mick Parker Nathan Medd, John Ismay, vacancy Bulletin contributions Unelected Members Please refer to guide notes in this Bulletin for details of how to contribute and send your material to both of the following: Dipterists Digest Editor Peter Chandler Dipterists Bulletin Editor Darwyn Sumner Secretary 122, Link Road, Anstey, Charnwood, Leicestershire LE7 7BX. John Kramer Tel. 0116 212 5075 31 Ash Tree Road, Oadby, Leicester, Leicestershire, LE2 5TE. [email protected] [email protected] Assistant Editor Treasurer Judy Webb Howard Bentley 2 Dorchester Court, Blenheim Road, Kidlington, Oxon. OX5 2JT. 37, Biddenden Close, Bearsted, Maidstone, Kent. ME15 8JP Tel. 01865 377487 Tel. 01622 739452 [email protected] [email protected] Conservation Dipterists Digest contributions Robert Wolton Locks Park Farm, Hatherleigh, Oakhampton, Devon EX20 3LZ Dipterists Digest Editor Tel. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

In Dir Lower, Khyber Pakhtunkhwa, Pakistan
IAJPS 2019, 06 (10), 13512-13520 Abdul Baset et al ISSN 2349-7750 CODEN [USA]: IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES Available online at: http://www.iajps.com Research Article DIVERSITY OF CARPENTER BEE FAUNA (XYLOCOPA SPP.) IN DIR LOWER, KHYBER PAKHTUNKHWA, PAKISTAN Akbar Hussain1, Mohammad Attaullah2, Muhammad Ather Rafi3, Hamad Khan1, Abdul Waris4, Anwar Zeb5, Abdul Baset 6 1Department of Zoology, Shaheed Benazir Bhutto University, Sheringal, Dir Upper, Khyber Pakhtunkhwa, Pakistan 2Department of Zoology, Faculty of Biological Science, University of Malakand, Pakistan 3Department of Zoology, Women University Swabi, Pakistan, Director National Insect Museum, Islamabad, Pakistan 4Department of Biotechnology, Quaid-e-Azam University Islamabad, Pakistan 5Department of Zoology, Faculty of Science, Hazara University, Mansehra, Pakistan 6 Department of Zoology, Bacha Khan University, Charsadda, Pakistan *Corresponding author: [email protected] Abstract: This study was conducted at District Dir Lower, in north western Pakistan for the evaluation of diversity of Xylocopa spp. during March to September 2015. The study area was divided in 7 different localities namely Chakdara, Talash, Timergara, Jandol, Khal, Darmal and Lal Qilla. Higher Simpson’s index (1_D) values were calculated for Talash (0.7464) followed by Khal (0.7392), Chakdara and Jandol (same value 0.7366), Darmal (0.7268), Lal Qila (0.7244) and lowest value was calculated for Timergara (0.716). Divider and Scale method was used for the morphometric measurement of carpenter bees. Out of the total 321 specimens collected, four species namely, Xylocopa collaris, Xylocopa acutipennis, Xylocopa dissimilis and Xylocopa pubescens were identified. X. dissimilis was the abundant species recorded which represented 29.60 % of the total collection, while X. -
Red List of Endemic IUCN Red CITES Bern Bonn Georgia Species of the List Conventi Convention Caucasus on (CMS) 1
Latin name Georgian Name English name Red List of Endemic IUCN Red CITES Bern Bonn Georgia species of the list Conventi Convention Caucasus on (CMS) 1. Capra aegagrus niamori Wild Goat, Bezoar CR VU II Erxleben. Goat 2. Capra caucasica dasavleTkavkasiuri West Caucasian EN + EN Güldenstädt & jixvi Tur Pallas. 3. Capra aRmosavleTkavkasiuri East Caucasian VU + NT cylindricornis Blyth. jixvi Tur, Dagestan Tur 4. Capreolus capreolus evropuli Sveli European Roe LC Linnaeus. Deer 5. Gazella qurciki, jeirani Goitered Gazelle RE VU II subgutturosa Güldenstädt. 6. Rupicapra arCvi, fsiti Northern Chamois EN LC II rupicapra Linnaeus. 7. Cervus elaphus keTilSobili iremi Red Deer CR LC II I Linnaeus. 8. Sus scrofa gareuli Rori, taxi Eurasian Wild LC Linnaeus. Boar 9. Canis aureus tura Golden Jackal LC III Linnaeus. 10. Canis lupus mgeli Grey Wolf LC II II Linnaeus. 11. Nyctereutes enotisebri ZaRli Racoon Dog LC procyonoides Gray. 12. Vulpes vulpes mela Red Fox LC III Linnaeus. 13. Felis chaus lelianis kata Jungle Cat VU LC II Schreber. 14. Felis silvestris tyis kata Wild Cat LC II II Shreber. 15. Felis libyca Forster. velis kata Steppe Cat 16. Lynx lynx Linnaeus. focxveri Eurasian Lynx CR LC II 17. Panthera pardus jiqi Leopard CR NT I II Linnaeus. 18. Hyaena hyaena afTari Striped Hyaena CR NT Linnaeus. 19. Lutra lutra wavi Eurasian Otter, VU NT I II Linnaeus. Common Otter 20. Martes foina kldis kverna Stone Marten, LC III Erxleben. Beech Marten 21. Martes martes tyis kverna European Pine LC Linnaeus. Marten 22. Meles meles maCvi Eurasian Badger LC Linnaeus. 23. Mustela lutreola waula European Mink EN II Linnaeus. -

Amphibiaweb's Illustrated Amphibians of the Earth
AmphibiaWeb's Illustrated Amphibians of the Earth Created and Illustrated by the 2020-2021 AmphibiaWeb URAP Team: Alice Drozd, Arjun Mehta, Ash Reining, Kira Wiesinger, and Ann T. Chang This introduction to amphibians was written by University of California, Berkeley AmphibiaWeb Undergraduate Research Apprentices for people who love amphibians. Thank you to the many AmphibiaWeb apprentices over the last 21 years for their efforts. Edited by members of the AmphibiaWeb Steering Committee CC BY-NC-SA 2 Dedicated in loving memory of David B. Wake Founding Director of AmphibiaWeb (8 June 1936 - 29 April 2021) Dave Wake was a dedicated amphibian biologist who mentored and educated countless people. With the launch of AmphibiaWeb in 2000, Dave sought to bring the conservation science and basic fact-based biology of all amphibians to a single place where everyone could access the information freely. Until his last day, David remained a tirelessly dedicated scientist and ally of the amphibians of the world. 3 Table of Contents What are Amphibians? Their Characteristics ...................................................................................... 7 Orders of Amphibians.................................................................................... 7 Where are Amphibians? Where are Amphibians? ............................................................................... 9 What are Bioregions? ..................................................................................10 Conservation of Amphibians Why Save Amphibians? ............................................................................. -

GIS-Based Modelling Reveals the Fate of Antlion Habitats in the Deliblato Sands Danijel Ivajnšič1,2 & Dušan Devetak1
www.nature.com/scientificreports OPEN GIS-based modelling reveals the fate of antlion habitats in the Deliblato Sands Danijel Ivajnšič1,2 & Dušan Devetak1 The Deliblato Sands Special Nature Reserve (DSSNR; Vojvodina, Serbia) is facing a fast successional process. Open sand steppe habitats, considered as regional biodiversity hotspots, have drastically decreased over the last 25 years. This study combines multi-temporal and –spectral remotely sensed data, in-situ sampling techniques and geospatial modelling procedures to estimate and predict the potential development of open habitats and their biota from the perspective of antlions (Neuroptera, Myrmeleontidae). It was confrmed that vegetation density increased in all parts of the study area between 1992 and 2017. Climate change, manifested in the mean annual precipitation amount, signifcantly contributes to the speed of succession that could be completed within a 50-year period. Open grassland habitats could reach an alarming fragmentation rate by 2075 (covering 50 times less area than today), according to selected global climate models and emission scenarios (RCP4.5 and RCP8.5). However, M. trigrammus could probably survive in the DSSNR until the frst half of the century, but its subsequent fate is very uncertain. The information provided in this study can serve for efective management of sand steppes, and antlions should be considered important indicators for conservation monitoring and planning. Palaearctic grasslands are among the most threatened biomes on Earth, with one of them – the sand steppe - being the most endangered1,2. In Europe, sand steppes and dry grasslands have declined drastically in quality and extent, owing to agricultural intensifcation, aforestation and abandonment3–6. -

The Diptera of Lancashire and Cheshire: Craneflies and Winter Gnats
The Diptera of Lancashire and Cheshire: Craneflies and Winter Gnats by Phil Brighton 32, Wadeson Way, Croft, Warrington WA3 7JS [email protected] Version 1.1 26 November 2017 1 Summary This document provides a new checklist for the craneflies and winter gnats (Tipuloidea, Ptychopteridae and Trichoceridae) to extend the lists of the diptera of Lancashire and Cheshire first published by Kidd and Bindle in 1959. Overall statistics on recording activity are given by decade and hectad. Checklists are presented for each of the three Watsonian vice-counties 58, 59, and 60 detailing for each species the number of records, year of earliest and most recent record, and the number of hectads with records. A combined checklist showing distribution by the three vice-counties is also included, covering a total of 264 species, amounting to 75% of the current British checklist. Introduction This report is the third in a series to update and extend the partial checklist of the diptera of Lancashire and Cheshire published in 1959 by Leonard Kidd and Alan Brindle1. There were two previous updates, in 19642 and 19713. The previous reports in this series cover the soldierflies and allies4 and the Sepsidae5, the latter family not having been covered in Ref 1. The reader is referred to the first two reports for the background and rationale of these checklists, as well as the history of diptera recording and available data sources. The description of methodology is also kept to a minimum in the present report: only significant differences from the previous publications will be outlined. -

Bergmann's Rule in Larval Ant Lions
Ecological Entomology (2003) 28, 645–650 Bergmann’s rule in larval ant lions: testing the starvation resistance hypothesis AMY E. ARNETT andNICHOLAS J. GOTELLI Department of Biology, University of Vermont, U.S.A. Abstract. 1. Body size of the ant lion Myrmeleon immaculatus follows Bergmann’s rule – an increase in body size towards higher latitudes. The hypothesis that ant lion body size is larger in the north as an adaptation for starvation resistance was tested. 2. In a laboratory experiment testing starvation resistance, survivorship curves differed among 10 ant lion populations for both a starved and a fed treatment. 3. The average number of months survived by each population was correlated positively with latitude for both treatments. Across both treatments and all populations, large individuals survived longer than small individuals; however individuals from high latitudes had higher survivorship, even after factoring out variation due to initial body size. 4. These results suggest that starvation resistance may be an adaptation for coping with reduced prey availability in high latitudes. Starvation resistance may contribute to latitudinal gradients in body size of ant lions and other ectotherms. Key words. Ant lion, Bergmann’s rule, body size, latitudinal gradients, Myrmeleon immaculatus, starvation resistance. Introduction body size (Cushman et al., 1993). If food availability decreases at high latitudes, starvation resistance may be Bergmann’s rule – an increase in body size with latitude – is genetically based and promote large body size at high lati- a common geographic pattern that has been described for tudes. Size-dependent resistance to starvation is supported many taxa including birds (James, 1970; Graves, 1991), by many studies of both endotherms and ectotherms mammals (Boyce, 1978; Sand et al., 1995; Sharples et al., (Brodie, 1975; Kondoh, 1977; Boyce, 1978; Lindstedt & 1996), fish (L’Abe´e-Lund et al., 1989; Taylor & Gotelli, Boyce, 1985; Murphy, 1985; Cushman et al., 1993).