Calcium and Iron Oxide Reactivity Studies for Chemical Looping Applications of Clean Energy Conversion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Iron (III) Oxide Anhydrous
Material Safety Data Sheet Iron (III) Oxide Anhydrous MSDS# 11521 Section 1 - Chemical Product and Company Identification MSDS Name: Iron (III) Oxide Anhydrous Catalog Numbers: I116-3, I116-500 Synonyms: Ferric Oxide Red; Iron (III) Oxide; Iron Sesquioxide; Red Iron Oxide. Fisher Scientific Company Identification: One Reagent Lane Fair Lawn, NJ 07410 For information in the US, call: 201-796-7100 Emergency Number US: 201-796-7100 CHEMTREC Phone Number, US: 800-424-9300 Section 2 - Composition, Information on Ingredients ---------------------------------------- CAS#: 1309-37-1 Chemical Name: Iron (III) Oxide %: 100 EINECS#: 215-168-2 ---------------------------------------- Hazard Symbols: None listed Risk Phrases: None listed Section 3 - Hazards Identification EMERGENCY OVERVIEW Warning! May cause respiratory tract irritation. May cause mechanical eye and skin irritation. Inhalation of fumes may cause metal-fume fever. Causes severe digestive tract irritation with pain, nausea, vomiting and diarrhea. May corrode the digestive tract with hemorrhaging and possible shock. Target Organs: None. Potential Health Effects Eye: Dust may cause mechanical irritation. Skin: Dust may cause mechanical irritation. May cause severe and permanent damage to the digestive tract. May cause liver damage. Causes severe pain, Ingestion: nausea, vomiting, diarrhea, and shock. May cause hemorrhaging of the digestive tract. The toxicological properties of this substance have not been fully investigated. Dust is irritating to the respiratory tract. Inhalation of fumes may cause metal fume fever, which is characterized Inhalation: by flu-like symptoms with metallic taste, fever, chills, cough, weakness, chest pain, muscle pain and increased white blood cell count. Chronic: Chronic inhalation may cause effects similar to those of acute inhalation. -

Depositional Setting of Algoma-Type Banded Iron Formation Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok
Depositional Setting of Algoma-type Banded Iron Formation Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok To cite this version: Blandine Gourcerol, P Thurston, D Kontak, O Côté-Mantha, J Biczok. Depositional Setting of Algoma-type Banded Iron Formation. Precambrian Research, Elsevier, 2016. hal-02283951 HAL Id: hal-02283951 https://hal-brgm.archives-ouvertes.fr/hal-02283951 Submitted on 11 Sep 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Depositional Setting of Algoma-type Banded Iron Formation B. Gourcerol, P.C. Thurston, D.J. Kontak, O. Côté-Mantha, J. Biczok PII: S0301-9268(16)30108-5 DOI: http://dx.doi.org/10.1016/j.precamres.2016.04.019 Reference: PRECAM 4501 To appear in: Precambrian Research Received Date: 26 September 2015 Revised Date: 21 January 2016 Accepted Date: 30 April 2016 Please cite this article as: B. Gourcerol, P.C. Thurston, D.J. Kontak, O. Côté-Mantha, J. Biczok, Depositional Setting of Algoma-type Banded Iron Formation, Precambrian Research (2016), doi: http://dx.doi.org/10.1016/j.precamres. 2016.04.019 This is a PDF file of an unedited manuscript that has been accepted for publication. -

Earth Systems Science Grades 9-12
Earth Systems Science Grades 9-12 Lesson 2: The Irony of Rust The Earth can be considered a family of four major components; a biosphere, atmosphere, hydrosphere, and geosphere. Together, these interacting and all-encompassing subdivisions constitute the structure and dynamics of the entire Earth. These systems do not, and can not, stand alone. This Module demonstrates, at every grade level, the concept that one system depends on every other for molding the Earth into the world we know. For example, the biosphere could not effi ciently prosper as is without gas exchange from the atmosphere, liquid water from the hydrosphere, and food and other materials provided by the geosphere. Similarly, the other systems are signifi cantly affected by the biosphere in one way or another. This Module uses Earth’s systems to provide the ultimate lesson in teamwork. March 2006 2 JOURNEY THROUGH THE UNIVERSE Lesson 2: The Irony of Rust Lesson at a Glance Lesson Overview In this lesson, students will investigate the chemistry of rust—the forma- tion of iron oxide (Fe2O3)—within a modern context, by experimenting with the conditions under which iron oxide forms. Students will apply what they have learned to deduce the atmospheric chemistry at the time that the sediments, which eventually became common iron ore found in the United States and elsewhere, were deposited. Students will interpret the necessary formation conditions of this iron-bearing rock in the context of Earth’s geochemical history and the history of life on Earth. Lesson Duration Four 45-minute class periods plus 10 minutes a day for maintence and observation for two weeks Core Education Standards National Science Education Standards Standard B3: A large number of important reactions involve the transfer of either electrons (oxidation/reduction reactions) or hydrogen ions (acid/base reactions) between reacting ions, molecules, or atoms. -

Banded Iron Formations
Banded Iron Formations Cover Slide 1 What are Banded Iron Formations (BIFs)? • Large sedimentary structures Kalmina gorge banded iron (Gypsy Denise 2013, Creative Commons) BIFs were deposited in shallow marine troughs or basins. Deposits are tens of km long, several km wide and 150 – 600 m thick. Photo is of Kalmina gorge in the Pilbara (Karijini National Park, Hamersley Ranges) 2 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock Iron rich bands: hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). Iron poor bands: chert (fine‐grained quartz) and low iron oxide levels Rock sample from a BIF (Woudloper 2009, Creative Commons 1.0) Iron rich bands are composed of hematitie (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). The iron poor bands contain chert (fine‐grained quartz) with lesser amounts of iron oxide. 3 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock • Archaean and Proterozoic in age BIF formation through time (KG Budge 2020, public domain) BIFs were deposited for 2 billion years during the Archaean and Proterozoic. There was another short time of deposition during a Snowball Earth event. 4 Why are BIFs important? • Iron ore exports are Australia’s top earner, worth $61 billion in 2017‐2018 • Iron ore comes from enriched BIF deposits Rio Tinto iron ore shiploader in the Pilbara (C Hargrave, CSIRO Science Image) Australia is consistently the leading iron ore exporter in the world. We have large deposits where the iron‐poor chert bands have been leached away, leaving 40%‐60% iron. -

Combustion of Iron Wool – Student Sheet
Combustion of iron wool – Student sheet To study Iron is a metal. Iron wool is made up of thin strands of iron loosely bundled together. Your teacher has attached a piece of iron wool to a see-saw balance. At the other end of the see-saw is a piece of Plasticine. Iron wool can combust. Your teacher is going to make the iron wool combust by heating it. If there is a change in mass, the see-saw will either tip to the left or to the right. To discuss or to answer 1 What do you think will happen? ............................................................................................................................................................. 2 Why do you think this will happen? ............................................................................................................................................................. ............................................................................................................................................................. 3 What do you see happen when it is demonstrated? ............................................................................................................................................................. 4 Was your prediction correct? ............................................................................................................................................................. Nuffield Practical Work for Learning: Model-based Inquiry • Combustion of iron wool • Student sheets page 1 of 4 © Nuffield Foundation 2013 • downloaded from -

The Combination of Calcium Oxide and Cu/Zro2 Catalyst and Their Selective Products for CO2 Hydrogenation
Article The Combination of Calcium Oxide and Cu/ZrO2 Catalyst and their Selective Products for CO2 Hydrogenation Soipatta Soisuwan1,a,b,*, Wilasinee Wisaijorn1, Chalida Nimnul1, Orawan Maunghimapan1, and Piyasan Praserthdam2 1 Department of Chemical Engineering, Faculty of Engineering, Burapha University, 169 Long-Hard Bangsaen Road, Saensuk Sub-District, Muang District, Chonburi, 20130, Thailand 2 Center of Excellence in Catalysis and Catalytic Reaction Engineering, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Phayathai Road, Pathuwan District, Bangkok 10330, Thailand E-mail: [email protected] (Corresponding author), [email protected] Abstract. The catalytic activities of Cu/ZrO2_CaO catalysts were investigated on CO2 hydrogenation. The CO2 hydrogenations were carried out over combination of calcium oxide and Cu/ZrO2 catalyst. Two calcination temperatures were chosen at 300 and 650 oC according to thermal decomposition results. The catalysts were characterized by means of N2 adsorption-desorption, H2 temperature programmed reduction and X-ray diffraction. The CO2 hydrogenation under atmospheric pressure and at 250 oC was carried out over copper-based catalysts combined with calcium oxide namely i.e. Cu/ZrO 2 _CaO300, Cu/ZrO 2 _CaO650, Cu/ZrO2_Cu/CaO and Cu/ZrO2+CaO. The catalytic activities over all catalysts were consistent for 4 hours. The catalytic reaction rates over copper-based catalysts were in the range of 21.8 – 47.4 mol L-1 s-1 g cat-1. The modification of calcium oxide can improve the catalytic activity of copper-based catalysts to 47.4 mol L-1 s-1 g cat-1. The calcination temperature can cause a difference in active species that impact on product selectivity. -

Calcium Oxide
CALCIUM OXIDE Prepared at the 19th JECFA (1975), published in NMRS 55B (1976) and in FNP 52 (1992). Metals and arsenic specifications revised at the 59th JECFA (2002). An ADI ‘Not limited’ was established at the 9th JECFA (1965). SYNONYMS Lime; INS No. 529 DEFINITION Chemical names Calcium oxide C.A.S. number 1305-78-8 Chemical formula CaO Formula weight 56.08 Assay Not less than 95.0% after ignition DESCRIPTION Odourless, hard, white or greyish white masses or granules, or white to greyish white powder FUNCTIONAL USES Alkali, dough conditioner, yeast food CHARACTERISTICS IDENTIFICATION Solubility (Vol. 4) Slightly soluble in water, insoluble in ethanol, soluble in glycerol (Caution: Protect eyes when adding water) Reaction with water Moisten the sample with water; heat is generated (Caution: Protect eyes when adding water). Test for alkali The sample is alkaline to moistened litmus paper Test for calcium (Vol. 4) Passes test PURITY Loss on ignition (Vol. 4) Not more than 10% (1 g, about 800o to constant weight) Barium Not more than 0.03% Cautiously mix 1.5 g of the sample with 10 ml water, add 15 ml of dilute hydrochloric acid TS, dilute to 30 ml with water and filter. To 20 ml of the filtrate add 2 g of sodium acetate, 1 ml of dilute acetic acid TS and 0.5 ml of potassium chromate TS and allow to stand for 15 min. The turbidity of the solution is not greater than that of a control prepared by adding water to 0.3 ml of barium standard solution (1.779 g barium chloride in 1000 ml of water) to make to 20 ml, adding 2 g of sodium acetate, 1 ml of dilute acetic acid TS and 0.5 ml of potassium chromate TS and allowing to stand for 15 min. -
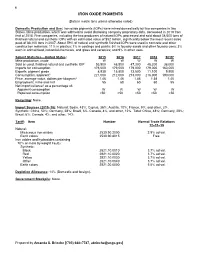
Iron Oxide Pigments Data Sheet
90 IRON OXIDE PIGMENTS (Data in metric tons unless otherwise noted) Domestic Production and Use: Iron oxide pigments (IOPs) were mined domestically by two companies in two States. Mine production, which was withheld to avoid disclosing company proprietary data, decreased in 2019 from that of 2018. Five companies, including the two producers of natural IOPs, processed and sold about 38,000 tons of finished natural and synthetic IOPs with an estimated value of $52 million, significantly below the most recent sales peak of 88,100 tons in 2007. About 59% of natural and synthetic finished IOPs were used in concrete and other construction materials; 11% in plastics; 7% in coatings and paints; 5% in foundry sands and other foundry uses; 3% each in animal food, industrial chemicals, and glass and ceramics; and 9% in other uses. Salient Statistics—United States: 2015 2016 2017 2018 2019e Mine production, crude W W W W W Sold or used, finished natural and synthetic IOP 53,500 48,500 47,300 48,200 38,000 Imports for consumption 176,000 179,000 179,000 179,000 160,000 Exports, pigment grade 8,930 15,800 13,500 11,100 9,900 Consumption, apparent1 221,000 212,000 213,000 216,000 190,000 Price, average value, dollars per kilogram2 1.46 1.46 1.46 1.58 1.40 Employment, mine and mill 55 60 60 60 55 Net import reliance3 as a percentage of: Apparent consumption W W W W W Reported consumption >50 >50 >50 >50 >50 Recycling: None. Import Sources (2015–18): Natural: Spain, 43%; Cyprus, 36%; Austria, 10%; France, 9%; and other, 2%. -

05/2203 Iron Oxide Extraction from Lunar and Martian Regoliths
05/2203 Iron oxide extraction from lunar and Martian regoliths Type of activity: Medium Study (4 months, 25 KEUR) Background The Reformer iron Sponge Cycle (RESC) has been proposed as a valid process to produce highly pure hydrogen from virtually any kind of hydrocarbon fuel on a bed of iron oxide (magnetite Fe3O4), present in the soils of the Moon and Mars. The process has been investigated in the frame of the Ariadna Call for Proposals 04/01 in view of future long-term manned lunar and Martian exploration. The process permits to operate a hydrogen-oxygen fuel cell for electrical power generation by producing hydrogen from either high energy density fuels brought from Earth or from fuels produced in situ (e.g. methane from the Sabatier process of from biomass decomposition). Fuels are converted into a mixture of hydrogen and carbon monoxide in a reforming reactor. In the following Iron Sponge Cycle iron oxide (magnetite/wuestite) is initially reduced by hydrogen and carbon monoxide to a lower oxide or down to iron metal with production of water steam and carbon dioxide. In a second step water steam is passed on the formed iron metal bed; iron oxide is replenished and water steam is reduced to highly pure hydrogen which can be fed without further purification treatments to the anodic compartment of a fuel cell for electrical power generation. The contact mass (iron/iron oxide) properties as size, porosity and composition of the pellets are very critical. The use of pure iron would theoretically maximise the efficiency of the cycle; the lifetime of the pellets is however limited by sintering effects. -

Iron Oxide Pigments
IRON OXIDE PIGMENTS By Michael J. Potter Domestic survey data and tables were prepared by Richelle J. Ellis, statistical assistant, and the world production table was prepared by Regina R. Coleman, international data coordinator. Natural iron oxides are derived from hematite, which is a red not included in tables 1, 2, and 4. iron oxide mineral; limonites, which vary from yellow to brown, Bayer Corp. introduced two new yellow grades of synthetic such as ochers, siennas, and umbers; and magnetite, which is IOPs, which were geared toward use in standard solvent-based black iron oxide. Synthetic iron oxide pigments are produced paints and coatings, as well as high solids and water-based from basic chemicals. The three major methods for the paints. One of the pigments was being manufactured at the manufacture of synthetic iron oxides are thermal decomposition company’s new iron oxide unit at New Martinsville, WV, and of iron salts or iron compounds, precipitation of iron salts the other yellow pigment was being made at Bayer’s facility usually accompanied by oxidation, and reduction of organic near Sao Paulo, Brazil, specifically for sale in North American compounds by iron (Podolsky and Keller, 1994, p. 765, 772). markets (Bayer Corp., October 23, 2000, Bayer Corp. introduces two new yellow grades of synthetic iron oxide Production pigments for use in architectural paints and coatings, accessed June 21, 2001, at URL http://www.bayerus.com/new/2000/ U.S. output of finished natural (mined) iron oxide pigments 10.23.00.html). (IOPs) sold by processors in 2000 was 87,800 metric tons (t), Laporte plc announced its intention to sell its pigments and about 5% less than in 1999; this category accounted for 51% of additives companies to K-L Holdings Inc., a company the tonnage and 15% of the value of total IOP output. -

Interaction of Water with the Cao(001) Surface † ‡ † § ‡ † ∥ Yuichi Fujimori, Xunhua Zhao, Xiang Shao, , Sergey V
Article pubs.acs.org/JPCC Interaction of Water with the CaO(001) Surface † ‡ † § ‡ † ∥ Yuichi Fujimori, Xunhua Zhao, Xiang Shao, , Sergey V. Levchenko, Niklas Nilius, , † ⊥ † Martin Sterrer,*, , and Hans-Joachim Freund † ‡ Department of Chemical Physics and Theory Department, Fritz-Haber-Institut der Max-Planck-Gesellschaft, Faradayweg 4-6, 14195 Berlin, Germany ∥ Institute of Physics, Carl-von-Ossietzky University, 26111 Oldenburg, Germany ⊥ Institute of Physics, University of Graz, Universitatsplatz̈ 5, 8010 Graz, Austria *S Supporting Information ABSTRACT: The interaction of water with the CaO(001) surface has been studied from ultrahigh-vacuum to submillibar water vapor pressures and at temperatures of 100 and 300 K using well-structured CaO(001)/Mo(001) thin-film model systems. Infrared reflection absorption spectroscopy (IRAS), X-ray photoelectron spectroscopy (XPS), and scanning tunneling microscopy (STM) in combination with density functional theory (DFT) calculations have been employed to reveal the correlation between the vibrational frequencies of the hydroxyl groups and the distinct hydroxylated surface phases that develop as a function of water exposure. In the low-coverage regime, water monomers, small water clusters, and one-dimensional water chains are formed on the CaO(001) surface. At increasing water coverages, water-induced structural disorder is observed, indicating 2+ partial solvation of Ca ions. Partial transformation of CaO(001) into a Ca(OH)2-like phase occurs upon dosing of water at submillibar water pressure. In addition, it was found that interfacial hydroxyl groups formed on the CaO(001) surface during water exposure at temperatures as low as 100 K shift the temperatures for ice desorption and for the transition from amorphous solid water to crystalline ice by 10 K as compared to those for the nonhydroxylated MgO surface. -

Analyses of High-Iron Sedimentary Bedrock and Diagenetic Features Observed with Chemcam at Vera Rubin Ridge, Gale Crater, Mars: Calibration and Characterization G
Analyses of High-Iron Sedimentary Bedrock and Diagenetic Features Observed With ChemCam at Vera Rubin Ridge, Gale Crater, Mars: Calibration and Characterization G. David, A. Cousin, O. Forni, P.-y. Meslin, E. Dehouck, N. Mangold, J. l’Haridon, W. Rapin, O. Gasnault, J. R. Johnson, et al. To cite this version: G. David, A. Cousin, O. Forni, P.-y. Meslin, E. Dehouck, et al.. Analyses of High-Iron Sedimentary Bedrock and Diagenetic Features Observed With ChemCam at Vera Rubin Ridge, Gale Crater, Mars: Calibration and Characterization. Journal of Geophysical Research. Planets, Wiley-Blackwell, 2020, 125 (10), 10.1029/2019JE006314. hal-03093150 HAL Id: hal-03093150 https://hal.archives-ouvertes.fr/hal-03093150 Submitted on 16 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. David Gaël (Orcid ID: 0000-0002-2719-1586) Cousin Agnès (Orcid ID: 0000-0001-7823-7794) Forni Olivier (Orcid ID: 0000-0001-6772-9689) Meslin Pierre-Yves (Orcid ID: 0000-0002-0703-3951) Dehouck Erwin (Orcid ID: 0000-0002-1368-4494) Mangold Nicolas (Orcid ID: 0000-0002-0022-0631) Rapin William (Orcid ID: 0000-0003-4660-8006) Gasnault Olivier (Orcid ID: 0000-0002-6979-9012) Johnson Jeffrey, R.