Botanica Orientalis, 2011 1.1.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Service Center, District-Haridwar
VLEs Details -Common Service Center, District-Haridwar SN District Tehsil Block VLE Name Contact Number Panchayat Address 1 HARIDWAR Laksar bhagwanpur Ajeet Singh 8650594978 Bhurna Laksar Laksar 2 HARIDWAR Hardwar Bhadrabad Sumit Tiwari 9045000108 \N Raamgarh Bheemgodakhadkhadiharidwaruttarakhand 3 HARIDWAR Roorkee laksar Bindu 9410710758 Raheempur 4 HARIDWAR Roorkee laksar Alok Kumar 8909464540 Imlikhera Imlikhera Dharampur Roorkee 5 HARIDWAR Roorkee narsan Praveen Kumar 9837194900 Sherpur Khelmau 6 HARIDWAR Roorkee Roorkee Anuj Kumar 9639829870 Bajuhedi mooldaspur roorkee 7 HARIDWAR Roorkee Roorkee Amit Kumar 8560579592 Mooldaspur 8 HARIDWAR Roorkee Bhadrabad Alka 9720860085 Mandawali Mandawali Narsan 9 HARIDWAR Roorkee Bhadrabad Deepak Kumar Singh 8433407886 Paniyala Chandapur Paniyala Chandpur 10 HARIDWAR Roorkee bhagwanpur Sonu Kumar 9927141508 Sikanderpur Bhainswal SIKANDERPUR ROAD PRIMARI SCHOOL SIKANDERPUR 11 HARIDWAR Laksar Roorkee Devender Kumar 9758692310 Dhadheki Dhana Laksar 12 HARIDWAR Laksar narsan Ajeet Kumar 7617642205 Kanewali Raisingh 13 HARIDWAR Roorkee Bhadrabad Vipin Kumar Agarwal 7535970405 \N 7 Civil Lines 7 Civil Lines Roorkee 14 HARIDWAR Laksar Laksar MANISH KUMAR 7351234344 Munda Khera Khurd SOCIETY ROAD LAKSAR 15 HARIDWAR Roorkee Bhadrabad Bhupendra Singh 9520378210 \N Left Canal Road 13/2 Left Canal Road Roorkee 16 HARIDWAR Roorkee laksar Sandeep Kumar Saini 9837543618 Khatka Roorkee Roorkee 17 HARIDWAR Roorkee Roorkee Jishan Ali 9837376973 Jainpur Jhanjheri JAINPUR JHANJHERI JAINPUR JHANJHERI 18 HARIDWAR -

Review of Research Impact Factor : 5.2331(Uif) Ugc Approved Journal No
Review Of ReseaRch impact factOR : 5.2331(Uif) UGc appROved JOURnal nO. 48514 issn: 2249-894X vOlUme - 7 | issUe - 10 | JUly - 2018 __________________________________________________________________________________________________________________________ ECOTOURISM AESTHETICS AND PROSPECTS: A GEOSPATIAL ASSESSMENT OF RAJAJI NATIONAL PARK Shairy Chaudhary1, M. S. Negi2 and Atul Kumar3 1&3Ph.D. Research Scholar, Associate Professor2 Department of Geography, H.N.B. Garhwal University (A Central University), Srinagar Garhwal, Uttarakhand, India. ABSTRACT There are 32 National parks, 92 Wild life sanctuaries located in 11 Himalayan states of India. Uttarakhand is the northern Himalayan state of India, where 6 National parks and 6 wild life sanctuaries established by the national and international organizations. These sites are well preserved, most beautiful attractions nationally and internationally among the tourists community for their amusement, knowledge and awareness regarding conservation of natural heritage. Rajaji National Park is one of the famous for his natural beauty, the prosperous diversity of flora, fauna and topographic landscape, which is located between Latitude 29° 56 ’ 40” N to 30° 20’ N and Longitude790 80’ E to 780 01’ 15” E in Pauri, Haridwar and Dehradun districts. It occupies around 820 Km2 areas in 9 forest ranges and situated in the lower Shiwalik range, foothills and Gangetic plains. Terrain relief of the park ranges between 271 m to 1381 m. from mean sea level. Shiwalik range passes from east to west from the park and River Ganga flows from North South and cut Shiwalik range in North East part of the park and makes flood plain in Southern part of Park. In the present study various aspects of the park such as topography, vegetative cover and Species, fauna species, Climate, accommodation facilities, transport and tourist attractions have been described using Remote Sensing and GIS geospatial tools and techniques. -

Making Way: Securing the Chilla-Motichur Corridor to Protect
OCCASIONAL REPORT NO. 10 MAKING WAY Till recent past the elephant population of Chilla on the east bank of the Ganga and Motichur, on the west, was one with regular movement between these two forest ranges of Rajaji National Park. Securing the Chilla-Motichur Corridor This movement, at one point, came to a virual halt because of to protect elephants of Rajaji National Park manmade obstacles like the Chilla power channel, an Army ammunition dump and rehabilitation of Tehri dam evacuees. The Eds: Vivek Menon, PS Easa and AJT Johnsingh problem was compounded by accidents owing to the railway track that passes through the area as National highway (NH 72). This study looks at WTI’s initiative in both securing the corridor as well as eliminating rail-hit incidents. B-13, Second floor, Sector - 6, NOIDA - 201 301 Uttar Pradesh, India Tel: +91 120 4143900 (30 lines) Fax: +91 120 4143933 Email: [email protected], Website: www.wti.org.in Wildlife Trust of India (WTI), is a non-profit conservation organisation, committed to help conserve nature, especially endangered species and threatened habitats, in partnership with communities and governments. Its vision is the natural heritage of India is secure. Project Team Suggested Citation: Menon, V; Easa,P.S; Johnsingh, A.J.T (2010) ‘Making Ashok Kumar Way’ - Securing the Chilla-Motichur corridor to protect elephants of Rajaji National Park. Wildlife Trust of India, New Delhi. Vivek Menon Aniruddha Mookerjee Keywords: Encroachment, Degradation, Sand mining, Corridor, Khand Gaon, P.S Easa Rehabilitation, Rajaji National Park, Anil Kumar Singh The designations of geographical entities in this publication and the A J T Johnsingh presentation of the material do not imply the expression of any opinion whatsoever on the part of the authors or WTI concerning the legal status of any country, territory or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries Editorial Team All rights reserved. -

List of Candidates Shortlisted for Document Verification for One Year Diploma Apprenticeship Training in Uttarakhand Against Advt
List of Candidates shortlisted for Document Verification for One year Diploma Apprenticeship Training in Uttarakhand against Advt. No. 93/2021 for FY 2021-22 Corresp Profesional Registr Date Corresponde Sr. Disp. Middle Correspondence Correspondence Corresponde ondence Brach ation First Name Last Name Category Of Father Name nce State No. No. Name Address1 Address 2 nce City Pin Name No Birth Name Code SJVN9 NEAR 3/2021 MALHOTR 10/2/1 DEEPAK DEVIDAS 1 1876 Civil POOJA General KASHIPUR Uttarakhand 244713 000435 A 997 MALHOTRA BAKERY 17 PATEL NAGAR VILLAGE SJVN9 PUNDOLI BHUVANESH 3/2021 5/30/1 ,POST - KARANPRA 2 1877 Civil MANORAMA GAUR General WAR Uttarakhand 246487 000434 998 MALAI,DISTT- YAG PRASAD 81 CHAMOLI ,UTTARA SJVN9 KHIMA IN CARE OF MANDI GATE 3/2021 2/14/1 3 1878 Civil ARNAV PANDEY General NAND SHANKAR TEA BAREILLY HALDWANI Uttarakhand 263139 000434 999 PANDEY TRADERS ROAD 18 PATRAMPUR SJVN9 UDHAM SINGH ROAD, KIRISHI 3/2021 3/12/2 NAGAR, 4 1879 Civil DHEERAJ KUMAR General HARI SINGH UTPADAN JASPUR Uttarakhand 244712 000435 000 UTTARAKHAN MANDI SAMITI 19 D JASPU SJVN9 GANDHI 3/2021 12/3/1 SUNIL 44/42 NEW GRAM 5 1880 Civil DEEPAK BHALLA General DEHRADUN Uttarakhand 248001 000434 999 BHALLA PARK ROAD KANWALI 09 ROAD VILLAGE & SJVN9 P.O.- 3/2021 6/15/2 YASHVEER TEHSIL- 6 1881 Civil ASHUTOSH SINGH OBC NAGANGAON, BARKOT Uttarakhand 249141 000434 000 SINGH BARKOT BARKOT, 65 UTTARKASHI SJVN9 3/2021 11/10/ JAIKHOLA 7 1882 Civil OSHIN MASSEY General S K MASSEY BHIMTAL BHIMTAL Uttarakhand 263136 000435 1998 TALLITAL 58 SJVN9 3/2021 2/8/19 VILLAGE POST OFFICE TEHRI 8 1883 Civil MADHU CHAUHAN General VIJAY SINGH Uttarakhand 249161 000437 99 AIRARI SILKAKHAL GARHWAL 07 VILL. -

Selected Bibliography on Rajaji National Park
Bibliography on Rajaji National Park Envis Selected Bibliography on Rajaji National Park 1 RESEARCH PAPERS 001. Annon. 1960. Improvement in our sanctuaries. Cheetal. 2(2): 74-77. In this paper author describes the Kansaro Sanctuary (now a part of Rajaji National Park) in Dehradun forests, Uttar Pradesh. 002. Badola, R. 1998. Attitudes of local people towards conservation and alternatives to forest resources: a case study from the lower Himalayas. Biodiversity and Conservation. 7(10): 1245-1259. This paper examines the attitudes of local people living in and around the forest corridor linking the Rajaji and Corbett National Park, Northern India. Door to door surveys were carried out, and using fixed response questionnaires people were interviewed to examine their views towards conservation and proposed alternatives to the forest resources for reducing biomass demand from the forest. The study revealed that in the area the concept of conservation of forests is well supported. Nevertheless, people are extracting biomass from the corridor forest for their sustenance. The dependence of the people on the forest is due to lack of alternatives to the forest resources, inability of the people to produce alternatives from market, and in some cases it is habitual or traditional. In a situation where forest resources will not be available, people without any alternatives to forest resources are ready to agitate against such rules. People who oppose such decisions are not always dependent on the corridor forest but are antagonistic towards the forest department and want to use this opportunity to retaliate by stealing from the forest. Their former category of people are the ones for whom income generating activities would be important while the later category should be the targets of extension programs designed to establish permanent lines of dialogue with the forest department. -

MAP:Haridwar(Uttarakhand)
77°40'0"E 77°50'0"E 78°0'0"E 78°10'0"E 78°20'0"E 78°30'0"E HARIDWAR DISTRICT GEOGRAPHICAL AREA (UTTARAKHAND) KEY MAP D E H R A D U R N U P ± N A R A H G A A S R H CA-01 W CA-02 A L 30°10'0"N 30°10'0"N CA-03 M U R Z A R F NO E F IJ IV A R R B I N N A G A A L R O S D B E ihari gher H Gran Total Population within the Geographical Area as per 2011 t Bug R gawal a RD A 18.90 Lacs. (Approx) 2A ¤£NH-7 D U TotalGeographicalArea(Sq.KMs) No.ofChargeAreas N 2305 3 Khedi Shikohpur Charge Area Identification Tahsil Name UR *# CA-01 Dariyapur Dayalpur (A.H.) P N CA-02 Daluwala Khurd CA-03 Purwala Birsangpur d R a o U R r H HARA a C g I a N d a OT SA r Sikraudha u M 30°0'0"N *# ta M S LEGEND Chauli Shahbuddin Pur o *# K D R Chappersher Afganpur A *# T OWA OW 30°0'0"N RD Kotamurad Nagar T LANDMARKS S GAG Ibrahimpur Masahi *# AL *# Aneki Hetmapur EHR *# I /" DISTRICT HEAD QUARTER Sikanderpur BhainswalRaipur *# *# Shivdas Pur Urf Teliwala G *# Garh Salempur Mahdood Shahpur (Ct) *# HARDWAR (NPP) A *# d /" TAHSIL HEAD QUARTER !. Bhagwanpur (Ct) a RHW Sirchandi o !."/ !. Rawali Mahdood (Ct) R *# !. s Khelpur Nasrullapur Bhagwanpu s !. -
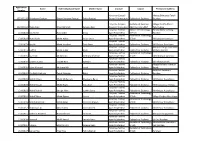
Application Number Name Father/Husband Name Mother Name Institute Course Permanent Address 1321001183 Anushyam Rastogi Shyam
Application Name Father/husband Name Mother Name Institute Course Permanent Address Number Haryana--Ganpati Vileege Bhimgoda Tehsil 1321001183 Anushyam Rastogi Shyam Narayan Rastogi Rekha Rastogi Group Of Institution Polytechnic Diploma Roorkee Haryana--Ganpati Bachelor of Business Village Akodha Khurd 1321001124 Reetu Devi Shyamlal Singh Suneeta Devi Group Of Institution Administration-BBA Tehsil Laksar Haryana--Techno Master of Technology- Purana Madarsa Marg 131000158 Aas Mohd Nasiruddin Anisa Apex Polytechnic M.Tech. Roorkee Haryana--Techno Bachelor of Technology- 131000169 Abdul Kadir Mohd Abbas Kaiser Jhain Apex Polytechnic B.Tech. Vill Iqbalpur Kamelpur Haryana--Techno 131000159 Ajaj Ali Mohd Jumshad Frida Bano Apex Polytechnic Polytechnic Diploma Vill Khelpur Nasullapur Haryana--Techno Rampur Chungi, Opp Grain 131000132 Gulfam Mohd Guljar Abda Apex Polytechnic Polytechnic Diploma Market, Roorkee Haryana--Techno Bachelor of Technology- 131000167 Javed Ali Mubarik Ali Mehruba Khatoon Apex Polytechnic B.Tech. Vill Kishanpur Jamalpur Haryana--Techno 131000165 Jonee K Kumar Loukee Ram Sulendri Apex Polytechnic Polytechnic Diploma Vill Dallawala Kala Haryana--Techno Village Nalhera Anantpur 131000164 Juber Ahamad Musharaf Ali Isarat Apex Polytechnic Polytechnic Diploma Roorki Haryana--Techno Kashipuri Sunehera, 131000131 Km Rakhi Kashyap Tejpal Kashyap Bably Apex Polytechnic Polytechnic Diploma Roorkee Haryana--Techno 131000162 Mohd Adnan Mohd Mohatram Shamsana Bano Apex Polytechnic Polytechnic Diploma Vill Khelpur Nasrullapur Haryana--Techno 131000156 Mohd Musab Mohd Ragib Noorzhan Apex Polytechnic Polytechnic Diploma Vill Bhagwanpur Khelpur Haryana--Techno 131000168 Mohd Rashid Jahagir Alam Julekha Bano Apex Polytechnic Polytechnic Diploma Vill Khelpur Nasrullapur Haryana--Techno Bachelor of Technology- 131000161 Mohd Takee Mohd Rijwan Nusrat Apex Polytechnic B.Tech. Village Khelpur Nasrullapur Haryana--Techno Bachelor of Technology- 131000160 Mohd Ahkam Ali Vahab Alam Sameena Begum Apex Polytechnic B.Tech. -
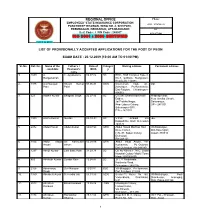
List of Provisionally Accepted Applications for the Post of Peon
REGIONAL OFFICE Phone: EMPLOYEES’ STATE INSURANCE CORPORATION 0135 – 2774762 / 63 PANCHDEEP BHAWAN, WING NO. 4, SHIVPURI, PREMNAGAR, DEHRADUN, UTTARAKHAND Fax Beat Code : I PIN Code : 248007 0135-2771542 ISO 9001 : 2000 CERTIFIED CHINTA SE MUKTI LIST OF PROVISIONALLY ACCEPTED APPLICATIONS FOR THE POST OF PEON EXAM DATE : 20.12.2009 (10:00 AM TO 01:00 PM) Sl. No. Ref. No. Name of the Father’s / Date of Categor Mailing address Permanent address candidate Husband’s Birth y name 1. 1859 A. C. Ayyaswamy 06.07.82 SC ESIC, Staff Complex Type-3, Ranganathan No-7, O-Block, Mangolpuri, New Delhi-110083 2. 1375 A.D.Narayan Shyam Kumar 01.06.83 GEN. C/o-Hetram Naik, AT- DO Patel Patel jhanakpur, Po-Baramkela, Dist-Raigarh, Chhattisgarh- 496551 3. 323 Aadhin Kumar Bhagwat Singh 20.07.86 SC C/o Shri Dharmendra Nath Pradeep Vihar, Dubey, Near Gavlira Chowk, Jai Prabha Nagar, Saharanpur, Near Labour Colony, UP – 247 001 Saharanpur (UP), PIN – 247 001 4. 1995 Aashu Kumar Gurdas 08-10-84 SC V.P.O. Ambadi Via -do- Dakpaththa Distt Dehradun 248125 5. 2072 Abdul Hamid Abdul shukur 08/07/90 GEN Abdul Hamid Marfhan Havi Vill-Barkanpur, Deve Camuli, Dist-Karemganj H.N.-97, Saken Colony, Assam-788810 Dehradun Pin-248121 6. 1104 Abdul Majid Sri Kalimullah 05.08.89 GEN Abdul Majid Ansari, Vill- Ansari Ansari Kurkawala, Po.-Doiwala, Dist-Dehradun, Pin-248140 7. 1297 Abhijit Kumar Late Babu Ram 31.03.78 SC Qtr.No-Reena F 7N.C, Jindal Hospital Colony Model Town Hisar-125005(H.R) 8. -

Page 1 of 72 COLLEGE of ENGINEERING ROORKEE (COER
COLLEGE OF ENGINEERING ROORKEE (COER) (Governed by Seth Roshan Lal Jain Trust) 7th KM on Roorkee – Haridwar Road Vardhman Puram Roorkee (Uttarakhand) August 22, 2020 Minutes of the meeting of the BOG of College of Engineering Roorkee (COER) held on 21st August 2020 at 12.00 Noon in online mode due to prevailing COVID pandemic. Following attended the meeting: 1. Er. JC Jain, Chairman, Seth Roshan Lal Jain Trust Chairman 2. Mrs. Sunita Jain, Vice Chairperson, Seth Roshan Lal Jain Trust Member 3. Dr. Subhash Jain, Secretary, Seth Roshan Lal Jain Trust Member 4. Mr. Shriyance Jain, Managing Director, Member Texplas Group of Industries, Trustee, Seth Roshan Lal Jain Trust 5. Mrs. Charu Jain, Trustee, Seth Roshan Lal Jain Trust Member 6. Professor SP Gupta, Director General, COER Member Secretary 7. Professor D. Ghosh, Head, E & CE Department, IIT Roorkee Member 8. Professor RP Saini, Alternate Hydro Energy Centre, IIT Roorkee Member 9. Dr. BM Singh, Director, COER Member 10. Dr. Pankaj Chaudhari, Associate Dean (Academics) Special Invitee 11. Dr. Siddharth Jain, Dean Research Special Invitee Nominations of two Members by the State Government are yet to be received. Dr. Anita Rawat, Member of the BoG and Registrar, Uttarakhand Technical University could not attend the meeting. Hon. Chairman welcomed all the members and thanked them for their gracious presence in the meeting of the BoG. He said COER looks forward to guidance from the Honourable members of this apex body. The suggestions made in the BoG are valuable and all steps are taken to implement them. For example, in the last BOG it was suggested that we increase intake in B.Tech. -

Haridwar District, Uttarakhand
क� द्र�यू�म भ जल बोड셍 जल संसाधन, नद� �वकास और गंगा संर�ण मंत्रालय भारत सरकार Central Ground Water Board Ministry of Water Resources, River Development and Ganga Rejuvenation Government of India Report on AQUIFER MAPPING AND GROUND WATER MANAGEMENT PLAN Haridwar District, Uttarakhand उ�रांचल �ेत्र, देहरादनू Uttaranchal Region, Dehradun CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENATION GOVERNMENT OF INDIA AQUIFER MAPPING REPORT HARIDWAR DISTRICT, UTTARAKHAND UTTARANCHAL REGION DEHRADUN OCTOBER – 2016 2 AQUIFER MAPPING REPORT, HARIDWAR DISTRICT, UTTARAKHAND By Vikas Tomar Assistant Hydrologist Preface Executive Summary CONTENTS Chapter 1: Introduction Objectives Scope of the Study Approach and Methodology Area Details Brief Description: Data availability, Data adequacy and Data gap analysis and Data generation Climate and Rainfall Climate Rainfall Physiography Structural Hills The Bhabar Geomorphology Land use Soil Drainage Agriculture and Cropping Pattern in Area Irrigation Geology Acknowledgement Chapter 2: Data Collection and Generation Hydrogeology Bhagwanpur Block Bahadrabad Block Roorkee Block Narsan Block Laksar Block Khanpur Block Long Term Depth to water Level Hydrochemistry Geophysical Data Acquisition Exploratory Drilling 3 Chapter 3: Data Interpretation, Integration and Aquifer Mapping Ground Water Exploration Chemical Qualities of Shallow Ground Water Geophysical Survey Results Interpretation Aquifer 3-D disposition and Lithological Cross Section of District Chapter 4: Ground Water Resources Assessment Of Ground Water Resource Potential Of Unconfined And Confined Aquifer System Computation of Static Ground Water Resource Confined Resource Methodology and Assumption Chapter 5: Ground Water Related Issues Decline in Groundwater Level Groundwater Quality Groundwater Demand and Budgeting Chapter 6: Management Strategies Conclusion Acknowledgement List of Tables:- 1. -
CSC E Governance Services Ltd
CSC e_Governance Services Ltd. Sr.No Agent Name Agent Address Dist name State name Pincode 1 Aayuesh Goel Kalsi Road Dakpathar Vikasnagar Dehradun Dehradun Uttarakhand 248125 Gandhi Bomma Center Road, Beside Vro Office, Alamuru, East 2 Achanta Srinivasarao East Godavari Andhra Pradesh 533233 Godavari, Ap 3 Ajay Joshi Pitgara, Badnawar, Dist. Dhar Dhar Madhya Pradesh 454660 4 Ajay Kumar Noukri Point, Rani Bajaar,Nohar Hanumangarh Rajasthan 335523 5 Akhilesh Thakur Court Road Dhar Dhar Madhya Pradesh 454446 4-125, Gandhi Center, Paritala, Kanchikacherla, Krishna, Andhra 6 Akula Narasimhaswamy Krishna Andhra Pradesh 521180 Pradesh 7 Anil Kumar Manjeri Csc Center,Poonthottathil Tower Nilambur Road Manjeri Malappuram Kerala 676121 8 Anil Namta Yashmehul Studio,Bagbera Colony J.P. Road East Singhbhum Jharkhand 831002 9 Ankit Saini Sarthak Computer Education Holi Tiba Tijara Alwar Rajasthan 301411 10 Arvind Madan Teli Near Bus Stand, Jalgaon Road, Jamner, Jalgaon Jalgaon Maharashtra 424206 11 Aziz Gohar Khan Tareen Vill Thapal, Najibabad Distt Bijnor Up Bijnor Uttar Pradesh 246763 12 Balwinder Singh Near Sub Tehsil, Alal Road Sherpur Sangrur Punjab 148025 13 Basavaraj Hiremath Maheshwar Digital,509, Main Road Pathade Galli Akkol-591211 Belgaum Karnataka 591211 14 Bhera Ram Prime Computers Opp. Rajasthan Marudhar Bank Bilara Jodhpur Jodhpur Rajasthan 342602 15 Chekka Suresh Kumar 7-135 Main Road Opp Sbi Rayavaram, East Godavari, Andhra Pradesh East Godavari Andhra Pradesh 533346 16 Chitikesu Harisuman H No 8-1-70 ,Opp.Mamatha Hospital , Jammikunta -
Nature and Science, 2009;7(2), ISSN 1545-0740, [email protected]
Nature and Science, 2009;7(2), ISSN 1545-0740, http://www.sciencepub.net, [email protected] Movement and Ranging Behaviour of Asian elephants Elephas maximus in and around the Rajaji National Park, North-West India Ritesh Joshi1 and Rambir Singh2 1G. B. Pant Institute of Himalayan Environment and Development, Garhwal Unit, Srinagar-Garhwal, 246 174, Uttarakhand, India 2SERC Division, Department of Science and Technology, Government of India, Technology Bhavan, New Delhi, 110 016, India [email protected] Short Title: Movement and Ranging Behaviour of Asian Elephant in North-West India. Abstract: The seasonal and annual movements and home ranges of two different recognized elephant herds and bull elephant were assessed by ground based data and direct observations in Chilla forest of the Rajaji National Park and its adjoining protected habitats from May 2005 to June 2007. During summer elephants use the lower slopes of Chilla forest but during monsoon their movement was towards upper areas of Luni, Rawasan and Pulani forest (south-east axis) and towards Shyampur and Chiriapur forests (south axis) and during the winter elephants again return back to plains of Ganges, where they utilize the riparian corridors. Large migration was observed during onset of summer (February-March). Herd’s movement was observed within an area of about 80 Km2 moving on the average less than 8 kilometers per day whereas bull elephant’s movement was observed to be about 390 Km2 moving on the average less than 27 kilometers per day. Home range sizes of herds varied between 13 Km2 (winter) and 24 Km2 (monsoon) and of bulls between 63 Km2 (winter) to 177 Km2 (monsoon).