Master Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Firth of Lorn Management Plan
FIRTH OF LORN MARINE SAC OF LORN MARINE SAC FIRTH ARGYLL MARINE SPECIAL AREAS OF CONSERVATION FIRTH OF LORN MANA MARINE SPECIAL AREA OF CONSERVATION GEMENT PLAN MANAGEMENT PLAN CONTENTS Executive Summary 1. Introduction CONTENTS The Habitats Directive 1.1 Argyll Marine SAC Management Forum 1.2 Aims of the Management Plan 1.3 2. Site Overview Site Description 2.1 Reasons for Designation: Rocky Reef Habitat and Communities 2.2 3. Management Objectives Conservation Objectives 3.1 Sustainable Economic Development Objectives 3.2 4. Activities and Management Measures Management of Fishing Activities 4.1 Benthic Dredging 4.1.1 Benthic Trawling 4.1.2 Creel Fishing 4.1.3 Bottom Set Tangle Nets 4.1.4 Shellfish Diving 4.1.5 Management of Gathering and Harvesting 4.2 Shellfish and Bait Collection 4.2.1 Harvesting/Collection of Seaweed 4.2.2 Management of Aquaculture Activities 4.3 Finfish Farming 4.3.1 Shellfish Farming 4.3.2 FIRTH OF LORN Management of Recreation and Tourism Activities 4.4 Anchoring and Mooring 4.4.1 Scuba Diving 4.4.2 Charter Boat Operations 4.4.3 Management of Effluent Discharges/Dumping 4.5 Trade Effluent 4.5.1 CONTENTS Sewage Effluent 4.5.2 Marine Littering and Dumping 4.5.3 Management of Shipping and Boat Maintenance 4.6 Commercial Marine Traffic 4.6.1 Boat Hull Maintenance and Antifoulant Use 4.6.2 Management of Coastal Development/Land-Use 4.7 Coastal Development 4.7.1 Agriculture 4.7.2 Forestry 4.7.3 Management of Scientific Research 4.8 Scientific Research 4.8.1 5. -

Greenland Barnacle 2003 Census Final
GREENLAND BARNACLE GEESE BRANTA LEUCOPSIS IN BRITAIN AND IRELAND: RESULTS OF THE INTERNATIONAL CENSUS, MARCH 2003 WWT Report Authors Jenny Worden, Carl Mitchell, Oscar Merne & Peter Cranswick March 2004 Published by: The Wildfowl & Wetlands Trust Slimbridge Gloucestershire GL2 7BT T 01453 891900 F 01453 891901 E [email protected] Reg. charity no. 1030884 © The Wildfowl & Wetlands Trust All rights reserved. No part of this document may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of WWT. This publication should be cited as: Worden, J, CR Mitchell, OJ Merne & PA Cranswick. 2004. Greenland Barnacle Geese Branta leucopsis in Britain and Ireland: results of the international census, March 2003 . The Wildfowl & Wetlands Trust, Slimbridge. gg CONTENTS Summary v 1 Introduction 6 2 Methods 7 3 Results 8 4 Discussion 13 4.1 Census total and accuracy 13 4.2 Long-term trend and distribution 13 4.3 Internationally and nationally important sites 17 4.4 Future recommendations 19 5 Acknowledgements 20 6 References 21 Appendices 22 ggg SUMMARY Between 1959 and 2003, eleven full international surveys of the Greenland population of Barnacle Geese have been conducted at wintering sites in Ireland and Scotland using a combination of aerial survey and ground counts. This report presents the results of the 2003 census, conducted between 27th and 31 March 2003 surveying a total of 323 islands and mainland sites along the west and north coasts of Scotland and Ireland. In Ireland, 30 sites were found to hold 9,034 Greenland Barnacle Geese and in Scotland, 35 sites were found to hold 47,256. -
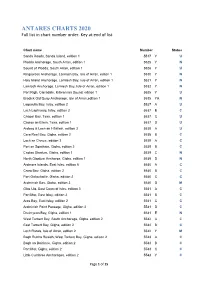
ANTARES CHARTS 2020 Full List in Chart Number Order
ANTARES CHARTS 2020 Full list in chart number order. Key at end of list Chart name Number Status Sanda Roads, Sanda Island, edition 1 5517 Y U Pladda Anchorage, South Arran, edition 1 5525 Y N Sound of Pladda, South Arran, edition 1 5526 Y U Kingscross Anchorage, Lamlash Bay, Isle of Arran, editon 1 5530 Y N Holy Island Anchorage, Lamlash Bay, Isle of Arran, edition 1 5531 Y N Lamlash Anchorage, Lamlash Bay, Isle of Arran, edition 1 5532 Y N Port Righ, Carradale, Kilbrannan Sound, edition 1 5535 Y U Brodick Old Quay Anchorage, Isle of Arran,edition 1 5535 YA N Lagavulin Bay, Islay, edition 2 5537 A U Loch Laphroaig, Islay, edition 2 5537 B C Chapel Bay, Texa, edition 1 5537 C U Caolas an Eilein, Texa, edition 1 5537 D U Ardbeg & Loch an t-Sailein, edition 3 5538 A U Cara Reef Bay, Gigha, edition 2 5538 B C Loch an Chnuic, edition 3 5539 A C Port an Sgiathain, Gigha, edition 2 5539 B C Caolas Gigalum, Gigha, edition 1 5539 C N North Gigalum Anchorge, Gigha, edition 1 5539 D N Ardmore Islands, East Islay, edition 5 5540 A C Craro Bay, Gigha, edition 2 5540 B C Port Gallochoille, Gigha, edition 2 5540 C C Ardminish Bay, Gigha, edition 3 5540 D M Glas Uig, East Coast of Islay, edition 3 5541 A C Port Mor, East Islay, edition 2 5541 B C Aros Bay, East Islay, edition 2 5541 C C Ardminish Point Passage, Gigha, edition 2 5541 D C Druimyeon Bay, Gigha, edition 1 5541 E N West Tarbert Bay, South Anchorage, Gigha, edition 2 5542 A C East Tarbert Bay, Gigha, edition 2 5542 B C Loch Ranza, Isle of Arran, edition 2 5542 Y M Bagh Rubha Ruaidh, West Tarbert -

The Clan Gillean
Ga-t, $. Mac % r /.'CTJ Digitized by the Internet Archive in 2012 with funding from National Library of Scotland http://archive.org/details/clangilleanwithpOOsinc THE CLAN GILLEAN. From a Photograph by Maull & Fox, a Piccadilly, London. Colonel Sir PITZROY DONALD MACLEAN, Bart, CB. Chief of the Clan. v- THE CLAN GILLEAN BY THE REV. A. MACLEAN SINCLAIR (Ehartottftcton HASZARD AND MOORE 1899 PREFACE. I have to thank Colonel Sir Fitzroy Donald Maclean, Baronet, C. B., Chief of the Clan Gillean, for copies of a large number of useful documents ; Mr. H. A. C. Maclean, London, for copies of valuable papers in the Coll Charter Chest ; and Mr. C. R. Morison, Aintuim, Mr. C. A. McVean, Kilfinichen, Mr. John Johnson, Coll, Mr. James Maclean, Greenock, and others, for collecting- and sending me genea- logical facts. I have also to thank a number of ladies and gentlemen for information about the families to which they themselves belong. I am under special obligations to Professor Magnus Maclean, Glasgow, and Mr. Peter Mac- lean, Secretary of the Maclean Association, for sending me such extracts as I needed from works to which I had no access in this country. It is only fair to state that of all the help I received the most valuable was from them. I am greatly indebted to Mr. John Maclean, Convener of the Finance Committee of the Maclean Association, for labouring faithfully to obtain information for me, and especially for his efforts to get the subscriptions needed to have the book pub- lished. I feel very much obliged to Mr. -
THE PLACE-NAMES of ARGYLL Other Works by H
/ THE LIBRARY OF THE UNIVERSITY OF CALIFORNIA LOS ANGELES THE PLACE-NAMES OF ARGYLL Other Works by H. Cameron Gillies^ M.D. Published by David Nutt, 57-59 Long Acre, London The Elements of Gaelic Grammar Second Edition considerably Enlarged Cloth, 3s. 6d. SOME PRESS NOTICES " We heartily commend this book."—Glasgow Herald. " Far and the best Gaelic Grammar."— News. " away Highland Of far more value than its price."—Oban Times. "Well hased in a study of the historical development of the language."—Scotsman. "Dr. Gillies' work is e.\cellent." — Frce»ia7is " Joiifnal. A work of outstanding value." — Highland Times. " Cannot fail to be of great utility." —Northern Chronicle. "Tha an Dotair coir air cur nan Gaidheal fo chomain nihoir."—Mactalla, Cape Breton. The Interpretation of Disease Part L The Meaning of Pain. Price is. nett. „ IL The Lessons of Acute Disease. Price is. neU. „ IIL Rest. Price is. nef/. " His treatise abounds in common sense."—British Medical Journal. "There is evidence that the author is a man who has not only read good books but has the power of thinking for himself, and of expressing the result of thought and reading in clear, strong prose. His subject is an interesting one, and full of difficulties both to the man of science and the moralist."—National Observer. "The busy practitioner will find a good deal of thought for his quiet moments in this work."— y^e Hospital Gazette. "Treated in an extremely able manner."-— The Bookman. "The attempt of a clear and original mind to explain and profit by the lessons of disease."— The Hospital. -

Download the .Pdf
Medieval Scotland: A Future for its Past Images © as noted in the text ScARF Summary Medieval Panel Document September 2012 i Medieval Scotland: a future for its past ScARF Summary Medieval Panel Report Mark Hall & Neil Price (eds) With panel contributions from: Colleen Batey, Alice Blackwell, Ewan Campbell, David Caldwell, Martin Carver, Piers Dixon, Richard Fawcett, Derek Hall, Heather James, Catherine Smith, Judith Stones, Sarah Thomas, Alex Woolf and Peter Yeoman. For contributions, images, feedback, critical comment and participation at workshops: Tom Addymann, Derek Alexander, Sue Anderson, Beverley Ballin Smith, David Bowler, Niall Brady, Stuart Campbell, Alison Cameron, Jonathon Cox, Barbara Crawford, Anne Crone, Stephen Driscoll, Katherine Forsyth, Sally Foster, Julie Franklin, Shannon Fraser, Strat Halliday, Jane Geddes, Martin Goldberg, Meggen Gondek, Moira Grieg, George Haggarty, Mhairi Hastie, David Hinton, Fraser Hunter, Alison Kyle, John Lawson, Chris Lowe, Hugh McBrien, Adrian Maldonado, Roger Mercer, Coralie Mills, Chris Morris, Charles Murray, Hilary Murray, Gordon Noble, Richard Oram, Tony Pollard, Niall Sharples, Douglas Speirs, Lisbeth Thoms, Marlo Willows and Sarah Winlow. ii Executive Summary Why research Medieval Scotland? Scotland’s medieval archaeology is extremely rich. Through its improved understanding, study and conservation, fascinating and critical aspects of the past can be explored, such as the development of towns, the arrival and integration of new peoples, how people farmed the land, and the development of political and religious systems. These multi-dimensional stories are played out at various levels: local, regional, national and international (principally but not solely European). Some of the issues and challenges faced by people then are equally familiar today, some will seem strange and exotic, and they fuse together in an intricate story that is the root from which modern Scotland has grown. -

Guide to Gaelic Origins of Place Names in Britain
Guide to Gaelic origins of place names in Britain NOTE: This booklet is one of a series of 4 booklets which cover the language origins of place names in Britain for Gaelic, Scandinavian, Scots and Welsh. The booklets can be downloaded from the Ordnance Survey web site at http://www.ordnancesurvey.co.uk as PDF files. November 2005 The background to the creation of this guide Early in the 20th century, Ordnance Survey issued a small pamphlet, The most common Gaelic words used on the Ordnance Survey Maps, for use with the one-inch maps of Scotland. In 1935, the Board of Celtic Studies and the University of Wales compiled a similar booklet, The most common Welsh words used on the Ordnance Survey Maps, for use with maps of Wales. This was reprinted in 1949. Later it was decided to increase the scope of the Gaelic names pamphlet, so the Scottish Geographical Society undertook a complete recompilation and published an enlarged edition in 1951 which included a list of Scandinavian elements used in Scottish place names. In 1968, Ordnance Survey produced an A5 booklet, Place names on maps of Scotland and Wales, which combined revised versions of both earlier publications. The booklet underwent several reprints, with the last reprint published in 1995. In the booklet, Ordnance Survey thanked Dr W F H Nicolaisen of the University of Edinburgh's School of Scottish Studies for his advice on the Gaelic and Scandinavian elements, William Matheson, senior lecturer in the Department of Celtic Studies at the University of Edinburgh for the revised guide to Gaelic pronunciation, and the Board of Celtic Studies of the University of Wales for their help with the Welsh glossary. -

NSA Special Qualities
Extract from: Scottish Natural Heritage (2010). The special qualities of the National Scenic Areas . SNH Commissioned Report No.374. The Special Qualities of the Scarba, Lunga and the Garvellachs National Scenic Area • Uninhabited, remote, wild islands • A seascape of distinctive and contrasting island groups • Solitude, sanctuary, reflection and retreat • Exceptional marine life • The notorious Corryvreckan and the Grey Dogs • The pyramidal island of Scarba • The irregularly-shaped Lunga, and its attendant islands and reefs • The low-lying slate island of Belnahua • The sloping and rocky Garvellachs Special Quality Further information • Uninhabited, remote, wild islands Set between the large mass of Jura and The South-West Argyll coast has innumerable off-shore the Argyll mainland, this arc of islands islands, islets, skerries, reefs and tide-swept rocky reefs and islands. These lie in a sea with powerful tidal currents presents a remote and isolated aspect. and deep waters. Lying west of the Slate Islands of Seil They are far removed from urban and Luing are the islands that comprise the NSA: centres, accessible only when sailing • The Garvellachs (four islands). conditions are favourable. • Lunga, the three Fiolas, Ormsa, Fladda and Belnahua, Eilean Dubh Beag and Mor. Today the there is no permanent habitation on any of the islands, although • The larger island of Scarba. the remains of past settlement show that it has not always been so. While there is still some grazing by sheep and cattle, the islands nowadays have a wild and undeveloped appearance with an absence of overt human influence that belies their history. • A seascape of distinctive and contrasting island groups These island groups are distinctive in the Despite their rocky and rough terrain, the Garvellachs’ south-west Argyll seascape, all with close-cropped green swards and distinctive hillsides of pink banded stone make a strong and surprising contrast interesting but contrasting rock with the Slate Isles dark coloured rocks. -

Re-Os Geochronology of the Neoproterozoic
Durham E-Theses Re-Os geochronology and geochemistry of Proterozoic sedimentary successions ROONEY, ALAN How to cite: ROONEY, ALAN (2011) Re-Os geochronology and geochemistry of Proterozoic sedimentary successions, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/621/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk Re-Os geochronology and geochemistry of Proterozoic sedimentary successions Alan David Rooney A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at Durham University Department of Earth Sciences, Durham University 2011 “All I am trying to do is help you understand that “The Name of the Rose” is merely a blip on an otherwise uninterrupted downward trajectory.” Abstract The Re-Os organic-rich sedimentary rocks (ORS) geochronometer has the potential to provide precise depositional ages and vital information on the Os isotope composition of palaeo-seawater. -

Notes on the Hawkweeds (Hieracium Sensu Lato) of Western Scotland
Watsonia, 8, 97-120 (1970). 97 Notes on the Hawkweeds (Hieracium sensu lato) of western Scotland A. G. KENNETH and A. McG. STIRLING Stronachullin, Ardrishaig, Argyll; 17 Austen Road, Jordanhill, Glasgow W3 ABSTRACT The distribution of species of Hieracium sensu stricto and the subspecies ofPilosella officinarum C. H. & F. W. Schultz in west and north-west Scotland is indicated for those areas and taxa of which the authors have personal knowledge. Corrections are included for the Distribution Maps of certain species as shown in the Critical Supplement. Ecological information is given for the species of Hieracium and their distribution is discussed in relation to the areas visited within each vice-county. A key to the genus Pilosella and an Index of Place Names are given as Appendices. INTRODUCTION Our knowledge of the distribution of the genus Hieracium in Scotland owes much to the explorations of certain English botanists in the period between 1850 and 1918. Notable among these were J. Backhouse Jnr, E. F. and W. R. Linton, Henry Groves, F. J. Hanbury, E. S. Marshall, W. A. Shoolbred and G. C. Druce, all of whom collected in the central and north western Highlands. After the first world war little attention was paid to this genus by British field botanists, due mainly to a lack of taxonomists competent to undertake determination of specimens. It was not until the commencement of the B.S.B.1. Distribution Maps Scheme in 1954 that they were encouraged by the intensive taxonomic study of the genus being undertaken by P. D. Sell and Dr C. -

Argyll Bird Report 25 2013
The Twenty Fifth ARGYLL BIRD REPORT With Systematic List for the year 2013 Edited by Jim Dickson Assisted by Robin Harvey and David Jardine Systematic List by John Bowler, Neil Brown, Malcolm Chattwood, Paul Daw, Jim Dickson, Bob Furness, Mike Harrison, David Jardine, Andy Robinson and Nigel Scriven ISSN 1363-4386 Copyright: Argyll Bird Club Feb. 2015 Argyll Bird Club Scottish Charity Number SC008782 Founded in 1985, the Argyll Bird Club aims to promote interest in and conservation of Argyll’s wild birds and their natural environment. The rich diversity of habitats in the county supports an exceptional variety of bird life. Many sites in Argyll are of international importance. The Club brings together people with varied experience, from complete beginners to experts, and from all walks of life. New members are particularly welcome. Activities Every spring and autumn there is a one-day meeting with illustrated talks and other activities. These meetings are held in conveniently central locations. Throughout the year there are field trips to local and more distant sites of interest. Publications The annual journal of the Club is the Argyll Bird Report, containing the Systematic List of all species recorded in the county during the year, together with reports and articles. The less formal quarterly newsletter, The Eider, gives details of forthcoming events and activities, reports of recent meetings, bird sightings, field trips, articles, and shorter items by members and others. Website www.argyllbirdclub.org To apply for membership, please (photocopy and) complete the form below and send to our Membership Secretary: Sue Furness, The Cnoc, Tarbet, G83 7DG. -

Scotland Files/Malts Sailing.Pdf
Reprinted from Sailing Magazine Copyright 2008 Reproduction or reuse of this work without written permission is prohibited Cheers! Scotland’s rugged West Coast is a beautiful cruising ground to be savored with a fine Scotch By Adrian Morgan with photograpy by Gary Blake and Wendy Johnson SAILING 77 The Classic Malts Cruise fleet meets at Talisker, Skye. 76 June 2008 rom the Yachtsman’s Pilot to it, and wisely suggest sounding the channel Talia, a Discovery 55, powers on a windless day as it leaves Oban en route to the Isle of Skye with the Isle of Mull comes this with a dinghy. It is that which brings sailing Scotland’s mountainous mainland in the background. A Hallberg-Rassy sails in the distance, as seen through a classic example of West Coast people to these waters. When Ronald Faux, porthole aboard a classic clipper ship, below. Scotland navigation: “From researching a book called The West some years southeast steer with Lagavulin ago, let slip his intentions to one who loved F distillery bearing 315 degrees sailing this intriguing coast, he was told: “You to pass clear southwest of Ruadh Mor— must be mad. Do you want the place to fill up taking care not to mistake Ardbeg (another with people? This is the world’s finest sailing. whisky distillery) for it.” Having left the shoal For heaven’s sake don’t let the secret out.” patch to starboard it is then simply a case, It must have taken the genius of Douglas advises the pilot, of heading for the perches Adams’ cartographer from the Hitchhiker’s marking the narrow entrance to the bay… “so Guide to the Galaxy, Slartibartfast, to draw that only the letters ULIN are visible on the such a coastline, with so many crinkly bits, so wall to the right of the castle.” many places to hide a boat from the prevail- The castle is Dunyvaig, a 12th century ing winds.