Biomechanics of Human Stratum Corneum: Dry Skin Conditions, Tissue Damage and Alleviation a Dissertation Submitted to the Depar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enabling Sweat-Based Biosensors: Solving the Problem of Low
Enabling sweat-based biosensors: Solving the problem of low biomarker concentration in sweat A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biomedical Engineering of the College of Engineering & Applied Science by Andrew J. Jajack B.S., Biology, Wittenberg University, 2014 Committee Chairs: Jason C. Heikenfeld, Ph.D. and Chia-Ying Lin, Ph.D. Abstract Non-invasive, sweat biosensing will enable the development of an entirely new class of wearable devices capable of assessing health on a minute-to-minute basis. Every aspect of healthcare stands to benefit: prevention (activity tracking, stress-level monitoring, over-exertion alerting, dehydration warning), diagnosis (early-detection, new diagnostic techniques), and management (glucose tracking, drug-dose monitoring). Currently, blood is the gold standard for measuring the level of most biomarkers in the body. Unlike blood, sweat can be measured outside of the body with little inconvenience. While some biomarkers are produced in the sweat gland itself, most are produced elsewhere and must diffuse into sweat. These biomarkers come directly from blood or interstitial fluid which surrounds the sweat gland. However, a two-cell thick epithelium acts as barrier and dilutes most biomarkers in sweat. As a result, many biomarkers that would be useful to monitor are diluted in sweat to concentrations below what can be detected by current biosensors. This is a core challenge that must be overcome before the advantages of sweat biosensing can be fully realized. The objective of this dissertation is to develop methods of concentrating biomarkers in sweat to bring them into range of available biosensors. -

Curling Cuticles of the Great Toenails: a Case Report of Eponychogryphosis
Open Access Case Report DOI: 10.7759/cureus.3959 Curling Cuticles of the Great Toenails: A Case Report of Eponychogryphosis Philip R. Cohen 1 1. Dermatology, San Diego Family Dermatology, San Diego, USA Corresponding author: Philip R. Cohen, [email protected] Abstract The cuticle, also referred to as the eponychium, creates a seal between the proximal nail fold and the nail plate. It is derived from both the ventral and dorsal portions of the proximal nail fold. In addition to its principle function as a barrier preventing allergens, irritants and pathogens from entering the nail cul-de- sac, the cuticle can play a role as a model for evaluating the etiology and management of diseases that affect capillary microcirculation, provide a source of solid tissue for genetic disorder studies, and aid in the evaluation of patients in whom the diagnoses of either systemic scleroderma or dermatomyositis is being entertained. Curling cuticle is a distinctive and unique occurrence. The clinical features of a man with curling cuticles on the lateral portion of both great toes is described. Although a deficiency in personal hygiene may partially account for the clinical finding, the pathogenesis of this observation remains to be established. The term ‘eponychogryphosis’ is proposed to describe the alteration of the patient’s cuticles. Categories: Dermatology, Internal Medicine, Rheumatology Keywords: curl, curling, cuticle, eponychium, eponychogryphosis, fold, great, onychogryphosis, nail, toe Introduction The cuticle, also known as the eponychium, is an extension of the stratum corneum from the proximal nail fold [1-3]. It forms a seal that prevents allergens, irritants, and pathogens from entering the potential space between the distal skin of the digit and the nail plate [4-5]. -

Stratum Corneum Biomechanics
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/281637624 Stratum Corneum Biomechanics Chapter · March 2009 DOI: 10.3109/9781420070958.016 CITATION READS 1 319 4 authors, including: Anne Potter Gustavo S Luengo L'Oréal L'Oréal 17 PUBLICATIONS 115 CITATIONS 90 PUBLICATIONS 1,603 CITATIONS SEE PROFILE SEE PROFILE Roberto Santoprete L'Oréal 10 PUBLICATIONS 140 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Biosurface interactions View project Nano Tribology View project All content following this page was uploaded by Roberto Santoprete on 21 October 2018. The user has requested enhancement of the downloaded file. STRATUM CORNEUM BIOMECHANICS INTRODUCTION As the outermost layer of the skin, the stratum corneum participates in the functional properties of the skin (1). For some functions, i.e. photoprotection (2, 3) or barrier protection (4), it is well accepted that the stratum corneum plays the primordial role. Concerning the mechanical properties of the skin, the influence of the mechanical properties of the stratum corneum is also recognized (5,6), but its exact level of importance is still in debates as it doesn’t exist clear results in the literature. The stratum corneum could be considered as a composite material mainly made of corneocytes, intercellular lipids, corneodesmosomes, and other intercellular proteoglycannes. Such a complex material should be characterized in a multi-scale approach in order to relate mechanical -

Chapter 6 – the Integumentary System
Chapter 6 – The Integumentary System I. The Skin and Subcutaneous Tissue: The skin is the body’s largest organ, accounting for 15% of the body weight of adults. Please see the terms for Figure 6.1 on page ________. Be sure to recognize the picture for your exam. The skin consists of two layers: Epidermis (outer layer made of stratified squamous epithelium) and dermis (deeper layer made of connective tissue). Hypodermis is a layer of subcutaneous fat that lies below the two layers of the skin. Skin is classified as thick or thin based on the relative thickness of the epidermis. Thick skin covers the palms, soles, and surfaces of the fingers and toes. It has a thick stratum corneum, a layer of dead cells. Thick skin has sweat glands, but no hair follicles or sebaceous (oil) glands. Thin skin has a thin stratum corneum and possesses sweat glands, hair follicles, and sebaceous glands. A. Functions of the Skin 1. Resistance to trauma and infection – epidermal cells contain a tough protein called keratin that gives the skin durability. Also, skin is usually dry and slightly acidic to protect the body from bacteria & fungal infections. 2. Other barrier functions – it keeps out excess water and keeps the body from losing excess water. The epidermis also blocks harmful UV (ultraviolet) rays, thereby keeping cancer-causing radiation from deeper tissues. The skin keeps out harmful chemicals, but is permeable to several drugs and poisons. 3. Vitamin D synthesis – Vitamin D is needed for bone development and maintenance. 4. Sensation – The skin is equipped with many nerve endings that react to heat, cold, touch, texture, pressure, vibration and tissue injury. -

Structure and Function of the Skin
2 Structure and Function of the Skin Skin disease illustrates structure and function. Loss of or Chapter Contents defects in skin structure impair skin function. Skin dis ease is discussed in more detail in the other chapters. ● Epidermis ● Structure ● Other Cellular Components EPIDERMIS ● Dermal–Epidermal Junction – The Basement Membrane Zone ● Dermis ● Skin Appendages Key Points ● Subcutaneous Fat 1. Keratinocytes are the principal cell of the epidermis 2. Layers in ascending order: basal cell, stratum spinosum, stratum granulosum, stratum corneum 3. Basal cells are undifferentiated, proliferating cells Key Points 4. Stratum spinosum contains keratinocytes connected by desmosomes 1. The major function of the skin is as a barrier to maintain 5. Keratohyalin granules are seen in the stratum granulosum internal homeostasis 6. Stratum corneum is the major physical barrier 2. The epidermis is the major barrier of the skin 7. The number and size of melanosomes, not melanocytes, determine skin color 8. Langerhans cells are derived from bone marrow and are the skin’s first line of immunologic defense ABSTRACT 9. The basement membrane zone is the substrate for attach- ment of the epidermis to the dermis The skin is a large organ, weighing an average of 4 kg and 10. The four major ultrastructural regions of the basement covering an area of 2 m2. Its major function is to act as membrane zone include the hemidesmosomal plaque of a barrier against an inhospitable environment – to pro the basal keratinocyte, lamina lucida, lamina densa, and tect the body from the influences of the outside world. anchoring fibrils located in the sublamina densa region of The importance of the skin is well illustrated by the high the papillary dermis mortality rate associated with extensive loss of skin from burns. -
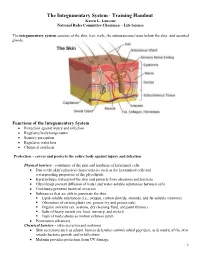
The Integumentary System - Training Handout Karen L
The Integumentary System - Training Handout Karen L. Lancour National Rules Committee Chairman – Life Science The integumentary system consists of the skin, hair, nails, the subcutaneous tissue below the skin, and assorted glands. Functions of the Integumentary System Protection against injury and infection Regulates body temperature Sensory perception Regulates water loss Chemical synthesis Protection – covers and protects the entire body against injury and infection Physical barriers - continuity of the skin and hardness of keratinzed cells Due to the skin’s physical characteristics such as the keratinized cells and waterproofing properties of the glycolipids. Keratin helps waterproof the skin and protects from abrasions and bacteria Glycolipids prevent diffusion of water and water-soluble substances between cells Continuity prevents bacterial invasion Substances that are able to penetrate the skin: . Lipid-soluble substances (i.e., oxygen, carbon dioxide, steroids, and fat-soluble vitamins) . Oleoresins of certain plants (ex. poison ivy and poison oak) . Organic solvents (ex. acetone, dry cleaning fluid, and paint thinner) . Salts of heavy metals (ex. lead, mercury, and nickel) . Topical medications as motion sickness patch Penetration enhancers Chemical barriers - (skin secretion and melanin) Skin secretions such as sebum, human defensins (antimicrobial peptides), acid mantle of the skin retards bacteria growth and/or kills them Melanin provides protection from UV damage 1 Skin secretions (acid mantle) Low pH and sebum slow bacterial growth on skin surface Human defensin – natural antibiotic Cathelicidins – proteins that prevent Strep A infection in wounded skin Melanin – chemical pigment that prevents UV damage Biological Barriers Langerhans’ cells, macrophages, and DNA Langerhans’ cells in epidermis present antigens to lymphocytes Dermal macrophages (2nd line of defense) – attack bacteria and viruses that have penetrated the epidermis Langerhan’s cells and macrophages present in the skin helps activate the body’s immune system. -

Skin, Very Close Up, Is Certain to Heighten Your Appreciation of This Most Obvious' of Organs
S~lnl Very Close Up I'm interested in how the skin works because it's the part ofmy body I'm mostfamiliar with, and I think the better I understand it, the better I can take care ofit. -Camille, 48, book publisher ntil you suffer a bad sunburn, have a close encounter Uwith a patch of poison ivy, or notice you don't quite look the way you'd like to look, you probably don't think much about your skin. If you're a runner, your joints are on your mind daily. If you lift weights, you visualize your muscle mass growing with each repetition. If your vision is changing, you probably fuss with decisions about your eyewear or laser eye surgery. But skin is an organ that, in the words of a retired surgeon I know, "we take for granted." Among the many reasons that we are able to take our skin for granted is the amazing reliability and durability of this important organ. Our skin weighs only about nine pounds but buttresses us against all manner of slings and arrows: sun, cold, a razor's edge, viruses, germs, and little bums. It varies in thickness with such remarkable preci sion that it can be flexible where needed (around the eyes, © Copyright 2000, David J. Leffell. MD. All rights reserved. 22 The Basics for example) and stiff and rigid where flexibility would be a handicap (the palms and soles). The skin on our eyelids is about half a millimeter thick (1 millimeter is about the thickness of the lead in a standard No.2 pencil). -

Accessory Structures of the Skin and Their Functions
Copyright EMAP Publishing 2020 This article is not for distribution except for journal club use Clinical Practice Keywords Skin/Hair/Nails/Sweat glands/Sebaceous glands Systems of life This article has been Skin double-blind peer reviewed In this article... l The four main accessory structures of the skin l Structure and function of hair and nails l The role of sweat and sebaceous glands Skin 2: accessory structures of the skin and their functions Key points Author Sandra Lawton is Queen’s Nurse, nurse consultant and clinical lead Accessory structures dermatology, The Rotherham NHS Foundation Trust. of the skin include the hair, nails, Abstract Understanding the skin requires knowledge of its accessory structures. sweat and These originate embryologically from the epidermis and include hair, nails, sweat sebaceous glands glands and sebaceous glands. All are important in the skin’s key functions, including protection, thermoregulation and its sensory roles. This article, the second in a Hair’s primary two-part series, looks at the structure and function of the main accessory structures functions are of the skin. protection, warmth and sensory Citation Lawton S (2020) Skin 2: accessory structures of the skin and their functions. reception Nursing Times [online]; 116; 1, 44-46. Nails protect the tips of the fingers ccessory structures of the skin l Distribution of sweat-gland products; and toes include the hair, nails, sweat l Psychosocial – hair plays an glands and sebaceous glands. important role in determining self The two main types AThese structures embryologi- image and social perceptions of sweat gland – cally originate from the epidermis and are (Bit.ly/RUAccessoryStructures; eccrine and apocrine often termed “appendages”; they can extend Kolarsick et al, 2011; Graham-Brown and – are responsible down through the dermis into the hypo- Bourke, 2006) . -

Skin Barrier Health: Regulation and Repair of the Stratum Corneum And
Skin Barrier Health: Regulation and Repair of the Stratum Corneum and the Role of Over-the-Counter Skin Care September 2016 | Volume 15 | Issue 9 | Original Article | 1047 | Copyright © 2016 Thomas Lee MD and Adam Friedman MD Department of Dermatology, George Washington School of Medicine and Health Sciences, Washington, DC Abstract The epidermis functions as a physical barrier that separates the inner body from the outside environment. The outermost layer of the epidermis, the stratum corneum, plays a key role in maintaining this barrier. There are numerous biochemical changes that take place to and in the keratinocyte as it migrates from the bottom, or startum basale, to the top layer of the epidermis in order for this barrier to function appropriately. In addition, external and internal factors, such as irritants and underlying medical diseases, can also affect the stratum corneum, both of which can potentially lead to disruption of barrier function and ultimately skin pathology. In this article, we will review keratinocyte biology as it relates to the formation and function of the stratum corneum. We will also review stratum corneum structure, physiology, and the impact of chemical agents and defective stratum corneum components that can lead to skin disease. Finally, we will briefly discuss how moisturizers repair defects in the stratum corneum and restore barrier function. J Drugs Dermatol. 2016;15(9):1047-1051. Keratinocyte Biology and Stratum Corneum Formation The epidermis is primarily made up of keratinocytes, in addition to melanocytes and Langerhans cells. It consists of four layers: the basal layer, spinous layer, granular layer, and cornified layer, which is also known as the stratum corneum. -

BIOL 2210L Unit 3: Integumentary System Authors: Terri Koontz and Megan Hancock, CNM Biology Department
1 BIOL 2210L Unit 3: Integumentary System Authors: Terri Koontz and Megan Hancock, CNM Biology Department Creative Commons Attribution-NonCommercial 4.0 International License Terms to Know for Unit 3 Layers of the Integument Accessory Structures of the Skin Additional Instructor Terms Epidermis Hair follicle Dermis Hair root Hypodermis Hair shaft Arrector pili muscle Cells of the Epidermis Sebaceous glands Keratinocytes sebum keratin Sweat glands Melanocytes Meissner’s corpuscle melanin Pacinian corpuscle Layers of the Epidermis Layers of the Dermis Stratum basale Papillary layer Stratum spinosum dermal papillae Stratum granulosum Reticular Layer Stratum lucidum Stratum corneum Membranes Serous Membranes Specific Serous Membranes Mucous membranes (Mucosae) Serosa Parietal pericardium Serous membranes (Serosae) Serous fluid Visceral pericardium Cutaneous membrane Parietal serosa Parietal pleura Visceral serosa Visceral pleura Parietal peritoneum Visceral peritoneum Learning Objectives (modified from HAPS learning outcomes) 1. Membranes (mucous, serous, cutaneous) a. Describe the structure and function of mucous, serous, and cutaneous membranes. b. Describe locations in the body where each type of membrane can be found. 2. General functions of the skin & the subcutaneous layer a. Describe the general functions of the skin. b. Describe the general functions of the subcutaneous layer (also known as the hypodermis). 3. Gross & microscopic anatomy of skin a. With respect to the epidermis: i. Identify and describe the tissue type making up the epidermis. 2 ii. Identify and describe the layers of the epidermis, indicating which are found in thin skin and which are found in thick skin. iii. Correlate the structure of thick and thin skin with the locations in the body where each are found. -

New Method of Measurement of Epidermal Turnover in Humans
cosmetics Article New Method of Measurement of Epidermal Turnover in Humans Kazuhisa Maeda ID School of Bioscience and Biotechnology, Tokyo University of Technology, 1404-1 Katakuramachi, Hachioji City, Tokyo 192-0982, Japan; [email protected]; Tel.: +81-42-637-2442 Received: 24 September 2017; Accepted: 7 November 2017; Published: 10 November 2017 Abstract: This report describes a new and simple technique to detect alterations in the rate of turnover in the epidermis without using any toxic chemical, such as a radiolabeled material. The method involves measuring the time course of the decrease of darkening of an ultraviolet A-irradiated site, compared with a non-irradiated control site. The turnover time of the persistent pigmentation on the inner side of the male forearm was 36.2 ± 6.2 days (age: 37.3 ± 11.3 years, mean ± standard deviation, n = 6), which is in reasonable agreement with the epidermal turnover time previously measured by injecting [3H] thymidine into human skin. Keywords: epidermal turnover; turnover time 1. Introduction Epidermal turnover is the process of the generation of keratinocytes in the basal layer of the epidermis and their eventual loss as corneocytes from the skin surface. The time taken for this process to be completed is called the epidermis turnover time or rate. This turnover plays an important role in maintenance of the skin’s barrier function. Melanin pigments, which are induced by ultraviolet (UV) exposure, are lost with the dead cells during epidermal turnover. If the turnover rate declines, they will remain apparent on the skin for longer. The rate of turnover is almost constant in healthy skin, and the stratum corneum transit time is approximately 20 days in young adults, although it gradually increases with age, lengthening by more than 10 days in older adults [1]. -

Table of Contents Previous Next Skin Is Composed of Two Layers, the Epidermis, the Outer Layer, Which Is Derived from Ectoderm
ATLAS OF HEAD AND NECK PATHOLOGY SKIN SKIN Skin is composed of two layers, the epidermis, the outer layer, which is derived from ectoderm, and the dermis, derived from mesoderm. The two layers are firmly adherent one to another with the dermis corresponding to the lamina propria of mucous membrane. Loose connective tissue lies under the dermis and corresponds to the superficial fascia. The epidermis is a stratified squamous keratinized epithelium with five layers or strata. (1) stratum germinativum or stratum basale lies just superficial to the dermis; (2) stratum spinosum or prickle cell layer; (3) stratum granulosum; (4) stratum lucidum; (5) stratum corneum. The stratum germinativum consists of a single layer of columnar or cuboidal cells. Mitotic figures are found here which produce new cells that eventually will be dis- placed into the next layer, the stratum spinosum, which is several layers thick and contains cells that are scale-like or polyhedral and which become flattened toward the surface. The name spinosum derives from “intercellular bridges” which appear to project from one cell to another but do not indicate cytoplasmic continuity between the cells The stratum spinosum and germinativum are grouped together as the malpighian layer and all cells here are referred to as keratinocytes. The stratum granulosum rests on top of the stratum spinosum and contains flattened polyhedral cells with vesicular nuclei. These cells contain granules of keratohyalin giving a granular appearance to the layer. The stratum lucidum is a clear translucent layer difficult to identify in many sections. The stratum corneum is the outermost layer of the epidermis and is composed of dead scale-like cells that become progressively flattened and joined together.