Chapter 6: the Preparation of the Squid Giant Synapse For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CEPHALOPODS 688 Cephalopods
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -

Long-Duration Anesthetization of Squid (Doryteuthis Pealeii) T
Marine and Freshwater Behaviour and Physiology Vol. 43, No. 4, July 2010, 297–303 Long-duration anesthetization of squid (Doryteuthis pealeii) T. Aran Mooneya,b*, Wu-Jung Leeb and Roger T. Hanlona aMarine Resources Center, Marine Biological Laboratory, Woods Hole, MA 02543, USA; bWoods Hole Oceanographic Institution, Woods Hole, MA 02543, USA (Received 4 May 2010; final version received 15 June 2010) Cephalopods, and particularly squid, play a central role in marine ecosystems and are a prime model animal in neuroscience. Yet, the capability to investigate these animals in vivo has been hampered by the inability to sedate them beyond several minutes. Here, we describe methods to anesthetize Doryteuthis pealeii, the longfin squid, noninvasively for up to 5 h using a 0.15 mol magnesium chloride (MgCl2) seawater solution. Sedation was mild, rapid (54 min), and the duration could be easily controlled by repeating anesthetic inductions. The sedation had no apparent effect on physiological evoked potentials recorded from nerve bundles within the statocyst system, suggesting the suitability of this solution as a sedating agent. This simple, long-duration anesthetic technique opens the possibility for longer in vivo investigations on this and related cephalopods, thus expanding potential neuroethological and ecophysiology research for a key marine invertebrate group. Keywords: anesthesia; sedation; squid; Loligo; neurophysiology; giant axon Introduction Although cephalopods are key oceanic organisms used extensively as experimental animals in a variety of research fields (Gilbert et al. 1990), there is a relative paucity of information on maintaining them under anesthesia for prolonged durations. Lack of established protocols for sedation beyond several minutes constrains Downloaded By: [Hanlon, Roger T.] At: 12:46 19 August 2010 experimental conditions for many neurobiological and physiological preparations. -
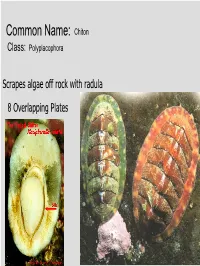
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Copyrighted Material
319 Index a oral cavity 195 guanocytes 228, 231, 233 accessory sex glands 125, 316 parasites 210–11 heart 235 acidophils 209, 254 pharynx 195, 197 hemocytes 236 acinar glands 304 podocytes 203–4 hemolymph 234–5, 236 acontia 68 pseudohearts 206, 208 immune system 236 air sacs 305 reproductive system 186, 214–17 life expectancy 222 alimentary canal see digestive setae 191–2 Malpighian tubules 232, 233 system taxonomy 185 musculoskeletal system amoebocytes testis 214 226–9 Cnidaria 70, 77 typhlosole 203 nephrocytes 233 Porifera 28 antennae nervous system 237–8 ampullae 10 Decapoda 278 ocelli 240 Annelida 185–218 Insecta 301, 315 oral cavity 230 blood vessels 206–8 Myriapoda 264, 275 ovary 238 body wall 189–94 aphodus 38 pedipalps 222–3 calciferous glands 197–200 apodemes 285 pharynx 230 ciliated funnel 204–5 apophallation 87–8 reproductive system 238–40 circulatory system 205–8 apopylar cell 26 respiratory system 236–7 clitellum 192–4 apopyle 38 silk glands 226, 242–3 coelomocytes 208–10 aquiferous system 21–2, 33–8 stercoral sac 231 crop 200–1 Arachnida 221–43 sucking stomach 230 cuticle 189 biomedical applications 222 taxonomy 221 diet 186–7 body wall 226–9 testis 239–40 digestive system 194–203 book lungs 236–7 tracheal tube system 237 dissection 187–9 brain 237 traded species 222 epidermis 189–91 chelicera 222, 229 venom gland 241–2 esophagus 197–200 circulatory system 234–6 walking legs 223 excretory system 203–5 COPYRIGHTEDconnective tissue 228–9 MATERIALzoonosis 222 ganglia 211–13 coxal glands 232, 233–4 archaeocytes 28–9 giant nerve -

Cephalopoda: Oegopsida)
J2 o Vol. 85, No. 16, pp. 205-222 30 August 1972 PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON FIRST RECORDS OF JUVENILE GIANT SQUID, ARCHITEVTHIS (CEPHALOPODA: OEGOPSIDA) By CLYDE F. E. ROPER AND RICHARD E. YOUNG Smithsonian Institution, Washington, D.C. 20560 and Department of Oceanography, University of Hawaii, Honolulu:, Hawaii 96822 The literature on cephalopods contains numerous records of individuals of the giant squid Architeuthis (see review in Clarke, 1966), the sole genus in the Architcuthidae. Most re- ports, of course, stress the large size of specimens, including the total length (a measure otherwise little used in cephalopod descriptions). Larvae and juveniles of Architeuthis, however, have remained unknown during the century following the original zoological recognition of the genus by Japetus Steen- strup (1857, et seq.). Two juvenile specimens of Architeuthis, representing sepa- rate species, were discovered in the collections of the In- stitute of Marine Sciences, University of Miami during studies on pelagic Cephalopoda. One specimen, 57 mm in mantle length (ML), was taken from the stomach of a fish, Alepi- saurus ferox (cf.), captured off Camara de Lobos, Madeira Island, Atlantic Ocean. The second specimen (45 mm ML) also was taken from the stomach of a fish, very probably Alcpisaurus (fide W. Klawe, personal communication), cap- tured by the R/V Shoyo Mam in the eastern Pacific off Chile. These specimens represent the smallest known individuals of Architeuthis; they are one order of magnitude smaller than the smallest previously reported specimen, an individual of A. physetcris of 460 mm VIL (Joubin, 1900). Iwai (1956) re- ported Architeuthis specimens of 92 and 104 mm ML, but both 16-PROC. -

Defensive Behaviors of Deep-Sea Squids: Ink Release, Body Patterning, and Arm Autotomy
Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in Charge: Professor Roy L. Caldwell, Chair Professor David R. Lindberg Professor George K. Roderick Dr. Bruce H. Robison Fall, 2009 Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy © 2009 by Stephanie Lynn Bush ABSTRACT Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair The deep sea is the largest habitat on Earth and holds the majority of its’ animal biomass. Due to the limitations of observing, capturing and studying these diverse and numerous organisms, little is known about them. The majority of deep-sea species are known only from net-caught specimens, therefore behavioral ecology and functional morphology were assumed. The advent of human operated vehicles (HOVs) and remotely operated vehicles (ROVs) have allowed scientists to make one-of-a-kind observations and test hypotheses about deep-sea organismal biology. Cephalopods are large, soft-bodied molluscs whose defenses center on crypsis. Individuals can rapidly change coloration (for background matching, mimicry, and disruptive coloration), skin texture, body postures, locomotion, and release ink to avoid recognition as prey or escape when camouflage fails. Squids, octopuses, and cuttlefishes rely on these visual defenses in shallow-water environments, but deep-sea cephalopods were thought to perform only a limited number of these behaviors because of their extremely low light surroundings. -

Synaptophysin Regulates Activity-Dependent Synapse Formation in Cultured Hippocampal Neurons
Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons Leila Tarsa and Yukiko Goda* Division of Biology, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0366 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved November 20, 2001 (received for review October 29, 2001) Synaptophysin is an abundant synaptic vesicle protein without a homogenotypic syp-mutant cultures, however, synapse forma- definite synaptic function. Here, we examined a role for synapto- tion is similar to that observed for wild-type cultures. Interest- physin in synapse formation in mixed genotype micro-island cul- ingly, the decrease in syp-mutant synapse formation is prevented tures of wild-type and synaptophysin-mutant hippocampal neu- when heterogenotypic cultures are grown in the presence of rons. We show that synaptophysin-mutant synapses are poor tetrodotoxin (TTX) or postsynaptic receptor blockers. Our donors of presynaptic terminals in the presence of competing results demonstrate a role for syp in activity-dependent com- wild-type inputs. In homogenotypic cultures, however, mutant petitive synapse formation. neurons display no apparent deficits in synapse formation com- pared with wild-type neurons. The reduced extent of synaptophy- Materials and Methods sin-mutant synapse formation relative to wild-type synapses in Hippocampal Cultures. Primary cultures of dissociated hippocam- mixed genotype cultures is attenuated by blockers of synaptic pal neurons were prepared from late embryonic (E18–19) or transmission. Our findings indicate that synaptophysin plays a newborn (P1) wild-type and syp-mutant mice as described (10). previously unsuspected role in regulating activity-dependent syn- Briefly, dissected hippocampi were incubated for 30 min in 20 apse formation. -

Flip Your Lid for Squid!
FLIP YOUR LID FOR SQUID! Adapted from http://njseagrant.org/wp-content/uploads/2014/03/squid_dissection.pdf PURPOSE Students learn the basics of squid anatomy and physiology by conducting a squid dissection. MATERIALS • frozen whole squid—not “clean” and not cut (get whole squid in frozen section of the grocery store) • hand lenses • dissection pan (plate) • scissors • tweezers • probe or toothpick • paper towels PROCEDURE Dissection of External Anatomy • Have students observe their squids for a few moments looking at the relationship between head and the rest of the body. Then ask students why they think squid are in the family of cephalopods (head-foots). • Tentacles and Arms: Have students count the number of arms. They may use the toothpicks to spread out the squid arms. Ask if all the arms look the same? There should be eight short arms and 2 long arms. Explain that the long thin arms with suckers only at the ends are called tentacles. The tentacles large ends with suckers are known as clubs. The tentacles are used to strike out and capture prey. The eight arms are used to hold onto prey when captured and bring food into its mouth. • Suction Cups: Student may use the magnifying lenses to get a closer look at the suction cups on each arm and tentacles. The suction cups have a small-toothed ring around each one and they are on short stalks. The suction cups are like suckers that help the arms hold onto the prey when it is captured and tries to escape. • Beak and Buccal Mass: Located within the circle of arms students can locate the squids mouth which is a beak. -

John Wilson Moore
John Wilson Moore BORN: Winston-Salem, North Carolina November 1, 1920 EDUCATION: Davidson College, B.S. Physics (1941) University of Virginia, Ph.D. Physics (1945) APPOINTMENTS: Radio Corporation of America Laboratories (1945–1946) Assistant Professor of Physics, Medical College of Virginia (1946–1950) Biophysicist, Naval Medical Research Institute (1950–1954) Associate Chief, Laboratory of Biophysics, NINDB, NIH (1954–1961) Professor of Physiology & Pharmacology, Duke University (1961–1988) Professor of Neurobiology, Duke University (1988–1990) Professor Emeritus of Neurobiology, Duke University (1990–present) HONORS AND AWARDS (SELECTED): Dupont Fellowship, University of Virginia (1941–1945) Fellow: National Neurological Research Foundation for Scientists (1961) Biophysical Society, Council and Executive Board (1966–1969; 1977–1979) Biomedical Engineering Society, Board of Directors (1971–1975) Trustee and Executive Committee, Marine Biological Laboratory (1971–1979; 1981–1985) K. S. Cole Award, Biophysical Society (1981) Fight for Sight Citation for Achievement in Basic Research (1982) John Wilson Moore initially became known for elucidating the action of tetrodotoxin and other neurotoxins using his innovative sucrose gap method for voltage clamping squid axon. He also was a pioneer in the nascent area of computational neuroscience, using computer simulations in parallel with experiments to predict experimental results and thus validate the concepts used in modeling. Intrigued by the possibility of applying his knowledge of physics to learn how neurons employ electricity to generate and transmit signals, he led the fi eld in exploring how ion channels and neuronal morphology affect excitation and signal propagation. He developed electronic instrumentation of high precision for electrophysiology, the result of experience gained through an unconventional career path: early training in physics, assignments involving feedback in the Manhattan Project, and learning principles of operational amplifi ers at the RCA Laboratories. -

Effects of Temperature on Escape Jetting in the Squid Loligo Opalescens
The Journal of Experimental Biology 203, 547–557 (2000) 547 Printed in Great Britain © The Company of Biologists Limited 2000 JEB2451 EFFECTS OF TEMPERATURE ON ESCAPE JETTING IN THE SQUID LOLIGO OPALESCENS H. NEUMEISTER*,§, B. RIPLEY*, T. PREUSS§ AND W. F. GILLY‡ Hopkins Marine Station of Stanford University, Department of Biological Science, 120 Ocean View Boulevard, Pacific Grove, 93950 CA, USA *Authors have contributed equally ‡Author for correspondence (e-mail: [email protected]) §Present address: Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461, USA Accepted 19 November 1999; published on WWW 17 January 2000 Summary In Loligo opalescens, a sudden visual stimulus (flash) giant and non-giant motor axons in isolated nerve–muscle elicits a stereotyped, short-latency escape response that is preparations failed to show the effects seen in vivo, i.e. controlled primarily by the giant axon system at 15 °C. We increased peak force and increased neural activity at low used this startle response as an assay to examine the effects temperature. Taken together, these results suggest that of acute temperature changes down to 6 °C on behavioral L. opalescens is able to compensate escape jetting and physiological aspects of escape jetting. In free- performance for the effects of acute temperature reduction. swimming squid, latency, distance traveled and peak A major portion of this compensation appears to occur in velocity for single escape jets all increased as temperature the central nervous system and involves alterations in the decreased. In restrained squid, intra-mantle pressure recruitment pattern of both the giant and non-giant axon transients during escape jets increased in latency, duration systems. -

Ommastrephidae 199
click for previous page Decapodiformes: Ommastrephidae 199 OMMASTREPHIDAE Flying squids iagnostic characters: Medium- to Dlarge-sized squids. Funnel locking appara- tus with a T-shaped groove. Paralarvae with fused tentacles. Arms with biserial suckers. Four rows of suckers on tentacular clubs (club dactylus with 8 sucker series in Illex). Hooks never present hooks never on arms or clubs. One of the ventral pair of arms present usually hectocotylized in males. Buccal connec- tives attach to dorsal borders of ventral arms. Gladius distinctive, slender. funnel locking apparatus with Habitat, biology, and fisheries: Oceanic and T-shaped groove neritic. This is one of the most widely distributed and conspicuous families of squids in the world. Most species are exploited commercially. Todarodes pacificus makes up the bulk of the squid landings in Japan (up to 600 000 t annually) and may comprise at least 1/2 the annual world catch of cephalopods.In various parts of the West- ern Central Atlantic, 6 species of ommastrephids currently are fished commercially or for bait, or have a potential for exploitation. Ommastrephids are powerful swimmers and some species form large schools. Some neritic species exhibit strong seasonal migrations, wherein they occur in huge numbers in inshore waters where they are accessable to fisheries activities. The large size of most species (commonly 30 to 50 cm total length and up to 120 cm total length) and the heavily mus- cled structure, make them ideal for human con- ventral view sumption. Similar families occurring in the area Onychoteuthidae: tentacular clubs with claw-like hooks; funnel locking apparatus a simple, straight groove.