The Association Between Threatened Miscarriage and Development of Gestational Hypertension/Pre-Eclampsia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association of Induced Abortion with Hypertensive Disorders Of
www.nature.com/scientificreports OPEN Association of induced abortion with hypertensive disorders of pregnancy risk among nulliparous women in China: a prospective cohort study Yinhua Su1,2, Xiaoping Xie3, Yanfang Zhou1, Hong Lin1, Yamei Li1, Na Feng1 & Jiayou Luo1* The relationship between induced abortion(IA) and hypertensive disorders of pregnancy(HDP) is inconclusive. Few studies have been conducted in China. In order to clarify the association between previous IA and risk of HDP, including gestational hypertension(GH) and pre-eclampsia(PE), we performed a community-based prospective cohort study enrolling 5191 eligible nulliparous women in selected 2 districts and 11 towns of Liuyang from 2013 to 2015. Multivariable logistic regression was conducted to examine whether IA was associated with HDP, GH and PE. Of the gravidea, 1378(26.5%) had a previous IA and 258(5.0%) diagnosed with HDP, including 141(2.7%) GH and 117(2.3%) PE. The diference in the incidence of GH and PE between gravidae having one versus those with two or more IAs was minimal. After adjustment for maternal age, body mass index at frst antenatal visit, education, virus infection and history of medical disorders, previous IA was signifcantly associated with HDP (OR = 0.67, 95%CI = 0.49 to 0.91) and PE (OR = 0.61, 95%CI = 0.38 to 0.97), but not with GH (OR = 0.73, 95%CI = 0.49 to 1.10). Additional adjustment for occupation, living area, anemia, gestational diabetes mellitus, psychological stress, conception climate and infant sex, multivariable analysis provided similar results. In conclusion, previous IA was associated with a lower risk of PE among nulliparous women. -

Incidence of Eclampsia with HELLP Syndrome and Associated Mortality in Latin America
International Journal of Gynecology and Obstetrics 129 (2015) 219–222 Contents lists available at ScienceDirect International Journal of Gynecology and Obstetrics journal homepage: www.elsevier.com/locate/ijgo CLINICAL ARTICLE Incidence of eclampsia with HELLP syndrome and associated mortality in Latin America Paulino Vigil-De Gracia a,⁎, José Rojas-Suarez b, Edwin Ramos c, Osvaldo Reyes d, Jorge Collantes e, Arelys Quintero f,ErasmoHuertasg, Andrés Calle h, Eduardo Turcios i,VicenteY.Chonj a Critical Care Unit, Department of Obstetrics and Gynecology, Complejo Hospitalario de la Caja de Seguro Social, Panama City, Panama b Critical Care Unit, Clínica de Maternidad Rafael Calvo, Cartagena, Colombia c Department of Gynecology and Obstetrics, Hospital Universitario Dr Luis Razetti, Barcelona, Venezuela d Unit of Research, Department of Gynecology and Obstetrics, Hospital Santo Tomás, Panama City, Panama e Department of Gynecology and Obstetrics, Hospital Regional de Cojamarca, Cajamarca, Peru f Department of Gynecology and Obstetrics, Hospital José Domingo de Obaldía, David, Panama g Unit of Perinatology, Department of Gynecology and Obstetrics, Instituto Nacional Materno Perinatal, Lima, Peru h Department of Gynecology and Obstetrics, Hospital Carlos Andrade Marín, Quito, Ecuador i Unit of Research, Department of Gynecology and Obstetrics, Hospital Primero de Mayo de Seguridad Social, San Salvador, El Salvador j Department of Gynecology and Obstetrics, Hospital Teodoro Maldonado Carbo, Guayaquil, Ecuador article info abstract Article history: Objective: To describe the maternal outcome among women with eclampsia with and without HELLP syndrome Received 7 July 2014 (hemolysis, elevated liver enzymes, and low platelet count). Methods: A cross-sectional study of women with Received in revised form 14 November 2014 eclampsia was undertaken in 14 maternity units in Latin America between January 1 and December 31, 2012. -
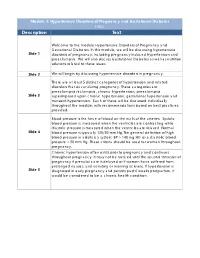
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Vitamin D, Pre-Eclampsia, and Preterm Birth Among Pregnancies at High Risk for Pre-Eclampsia: an Analysis of Data from a Low-Dos
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author BJOG. Author Manuscript Author manuscript; Manuscript Author available in PMC 2021 January 29. Published in final edited form as: BJOG. 2017 November ; 124(12): 1874–1882. doi:10.1111/1471-0528.14372. Vitamin D, pre-eclampsia, and preterm birth among pregnancies at high risk for pre-eclampsia: an analysis of data from a low- dose aspirin trial AD Gernanda, HN Simhanb, KM Bacac, S Caritisd, LM Bodnare aDepartment of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA bDivision of Maternal-Fetal Medicine, Magee-Women’s Hospital and Department of Obstetrics, Gynecology and Reproductive Sciences, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA cDepartment of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA dDepartment of Obstetrics, Gynecology and Reproductive Sciences and Department of Pediatrics, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA eDepartments of Epidemiology and Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh Graduate School of Public Health and School of Medicine, Pittsburgh, PA, USA Abstract Objective—To examine the relation between maternal vitamin D status and risk of pre-eclampsia and preterm birth in women at high risk for pre-eclampsia. Design—Analysis of prospectively collected data and blood samples from a trial of prenatal low- dose aspirin. Setting—Thirteen sites across the USA. Population—Women at high risk for pre-eclampsia. Methods—We measured 25-hydroxyvitamin D [25(OH)D] concentrations in stored maternal serum samples drawn at 12–26 weeks’ gestation (n = 822). We used mixed effects models to Correspondence: LM Bodnar, University of Pittsburgh Graduate School of Public Health, A742 Crabtree Hall, 130 DeSoto St, Pittsburgh, PA 15261, USA. -

Policy of Interventionist Versus Expectant Management of Severe
WHO recommendations Policy of interventionist versus expectant management of severe pre-eclampsia before term WHO recommendations Policy of interventionist versus expectant management of severe pre-eclampsia before term WHO recommendations: policy of interventionist versus expectant management of severe pre-eclampsia before term ISBN 978-92-4-155044-4 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. WHO recommendations: policy of interventionist versus expectant management of severe pre-eclampsia before term. Geneva: World Health Organization; 2018. -

Ophthalmic Associations in Pregnancy
CLINICAL Ophthalmic associations in pregnancy Queena Qin, Celia Chen, Sudha Cugati PREGNANCY RESULTS in various physiological variation in pregnancy.2 It normally changes in the female body, including in fades slowly after pregnancy and does the eyes. A typical pregnancy results in not need active intervention. Background A range of ocular pathology exists cardiovascular, pulmonary, metabolic, • Cornea – corneal thickness, curvature during pregnancy. Some pre-existing eye hormonal and immunological changes. and sensitivity may be altered during conditions, such as diabetic retinopathy, Hormonal changes occur, with a rise of pregnancy. Corneal thickness and can be exacerbated during pregnancy. oestrogen and progesterone levels to curvature can increase in pregnancy, Other conditions manifest for the first suppress the menstrual cycle.1 especially in the second and third time during pregnancy as a result of The eye, an end organ, undergoes trimesters, and return to normal in complications such as pre-eclampsia changes during pregnancy. Some of the postpartum period.3 Patients who and eclampsia. Early recognition and understanding of the management of these changes exacerbate pre-existing wear contact lenses may experience ophthalmic conditions is crucial. eye conditions, while other conditions intolerance to the use of contact lenses. manifest for the first time during Pregnant women should be advised Objective pregnancy. Early recognition and to delay obtaining a new prescription The aim of this article is to discuss the understanding of management of for glasses or undergoing a contact physiological and pathological changes in the eyes of pregnant women. ophthalmic conditions during pregnancy lens fitting until after delivery. Laser Pathological changes are sub-divided is crucial for the primary care physician. -

Pre-Eclampsia and High Blood Pressure During Pregnancy
Pre-eclampsia and High Blood Pressure During Pregnancy What is high blood pressure? A blood pressure measurement is usually recorded as two numbers, Blood pressure is the force that pushes against your blood vessel such as 120 over 80 (120/80). High blood pressure is also called walls each time your heart squeezes and relaxes to pump the blood hypertension. Hypertension is diagnosed when either the top or the through your body. Blood pressure measurement is a very useful bottom number is higher than normal. way to monitor the health of your cardiovascular system (heart and blood vessels). Why is blood pressure important develops, it does not go away until after the baby is born. Women with pre-eclampsia may require an earlier delivery, during pregnancy? either by labour induction or caesarean section, in order to During pregnancy, very high blood pressure (severe hypertension) protect the health of themselves and their baby. In some cases, can cause complications for both you and your baby, including: pre-eclampsia can develop after childbirth and you should • Poor growth of your baby – due to low nutrition and oxygen alert your doctor or midwife of any concerns you may have supply from the placenta after your baby is born. • Prematurity – if early delivery (before 37 weeks) is required to protect the health of you or your baby • Placental abruption – the placenta may prematurely separate from the wall of the uterus (womb), leading to bleeding and the need for an emergency birth in some cases • Pre-eclampsia – a condition involving high blood pressure and abnormal function in one or more organs during pregnancy What are the different types of high blood pressure that affect pregnant women? 1. -

Shoulder Dystocia Abnormal Placentation Umbilical Cord
Obstetric Emergencies Shoulder Dystocia Abnormal Placentation Umbilical Cord Prolapse Uterine Rupture TOLAC Diabetic Ketoacidosis Valerie Huwe, RNC-OB, MS, CNS & Meghan Duck RNC-OB, MS, CNS UCSF Benioff Children’s Hospital Outreach Services, Mission Bay Objectives .Highlight abnormal conditions that contribute to the severity of obstetric emergencies .Describe how nurses can implement recommended protocols, procedures, and guidelines during an OB emergency aimed to reduce patient harm .Identify safe-guards within hospital systems aimed to provide safe obstetric care .Identify triggers during childbirth that increase a women’s risk for Post Traumatic Stress Disorder and Postpartum Depression . Incorporate a multidisciplinary plan of care to optimize care for women with postpartum emergencies Obstetric Emergencies • Shoulder Dystocia • Abnormal Placentation • Umbilical Cord Prolapse • Uterine Rupture • TOLAC • Diabetic Ketoacidosis Risk-benefit analysis Balancing 2 Principles 1. Maternal ‒ Benefit should outweigh risk 2. Fetal ‒ Optimal outcome Case Presentation . 36 yo Hispanic woman G4 P3 to L&D for IOL .IVF Pregnancy .3 Prior vaginal births: 7.12, 8.1, 8.5 (NCB) .Late to care – EDC ~ 40-41 weeks .GDM Type A2 – somewhat uncontrolled .4’11’’ .Hx of Lupus .BMI 40 .Gained ~ 40 lbs during pregnancy Question: What complication is she a risk for? a) Placental abruption b) Thyroid Storm c) Preeclampsia with severe features d) Shoulder dystocia e) Uterine prolapse Case Presentation . 36 yo Hispanic woman G4 P3 to L&D for IOL .IVF Pregnancy .3 -

Study of the Placental Attachment of Funiculus
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(1):3535-40. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.107 STUDY OF THE PLACENTAL ATTACHMENT OF FUNICULUS UMBILICALIS IN NORMAL AND PRE-ECLAMPTIC PREGNANCIES AND ITS EFFECTS ON BIRTH WEIGHT Ankit Jain *1, Sonia Baweja 2, Rashmi Jain 3. *1 M.S., Ex. Resident, Department of Anatomy, Gandhi Medical College, Bhopal (M.P.), India. 2 M.S., Associate Professor, Department of Anatomy, Gandhi Medical College, Bhopal (M.P.), India. 3 M.D., Lab Head, Consultant Pathologist, SRL Diagnostics Ltd, Malviya Nagar, Bhopal (M.P.), India. ABSTRACT Introduction: Abnormalities in the insertion of umbilical cord is associated with a number of complications in pregnancy and these complications may adversely affect the fetus. The aim of this study was to evaluate the variations in the attachment of umbilical cord in normal and pre-eclamptic pregnancies and to assess the effects of variable cord insertions on fetal birth weight. Materials and Methods: Seventy placentae each of normotensive and pre-eclamptic pregnancies were studied (n=140). After delivery, weight of the baby was recorded by using weighing machine and the attachment of umbilical cord on placenta was observed. Results: In the present study, commonest site of insertion of umbilical cord was central (60%) in normal pregnancies, whereas in pre-eclamptic pregnancies, a common site of insertions of umbilical cord were central (37.14%) and/or eccentric (34.28%). Marginal cord insertions were found 2.11 times more in pre-eclamptic pregnancies as compared to normal pregnancies. -

Common Skin Changes During Pregnancy
EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY Information Leaflet for Patients COMMON SKIN CHANGES DURING PREGNANCY The aim of this leaflet This leaflet is designed to help you understand more about skin changes during pregnancy. It tells you about some of the most common, usually harmless but sometimes unpleasant skin changes, and explains what you can do to help them. COMMON SKIN What are common skin changes during pregnancy? Skin changes during pregnancy include: CHANGES 1. stretch marks (striae) DURING 2. skin tags 3. changes in hair growth PREGNANCY 4. spider veins and varicose veins 5. darkening of areas of your skin (melasma or cloasma) 6. pimple breakouts (acne) - information in a separate leaflet 7. darkening of moles and freckles - information in a separate leaflet. What are stretch marks (striae)? What are skin tags (fibroma Stretch marks (also called striae) are linear pendulum, acrochordon)? marks that most often develop over the Skin tags are very small, 1-5 mm, loose, breasts, abdomen, hips, and thighs. They polyp-like, skin-coloured growths of skin begin as reddish purple lines and with that usually appear under your arms or time, they become white atrophic (wrinkled) breasts. The increased appearance of skin scars. They are very common in pregnancy, tags during pregnancy is hormonally- occurring in up to 50% to 90% of pregnant induced in areas exposed to mechanical women. They are usually asymptomatic, but irritation. They may disappear after delivery. rarely may cause burning and itching. If they still persist, these tiny tags can be Stretch marks are caused by stretching removed by your dermatologist. -

Placental Abruption in Each Phenotype of Hypertensive Disorders of Pregnancy: a Retrospective Cohort Study Using a National Inpatient Database in Japan
Hypertension Research (2021) 44:250–252 https://doi.org/10.1038/s41440-020-00557-2 COMMENT Placental abruption in each phenotype of hypertensive disorders of pregnancy: a retrospective cohort study using a national inpatient database in Japan 1 1,2 2,3 Nelson Sass ● Gilberto Nagahama ● Henri Augusto Korkes Received: 24 August 2020 / Revised: 2 September 2020 / Accepted: 3 September 2020 / Published online: 7 October 2020 © The Japanese Society of Hypertension 2020 In a very interesting paper, Naruse et al. [1] analyzed a ret- Therefore, it is plausible to claim that PE is also a “bloody rospective cohort from a Japanese database, identifying a business” (Fig. 1). higher incidence of placental abruption in women with severe Advances in the knowledge of the pathological changes preeclampsia (PE) when admitted at less than 34 weeks associated with PE, especially in the early onset forms, gestational age (GA). Abruption of the placenta is an obstetric suggest that poor trophoblastic invasion in the early stages emergency that is usually catastrophic and requires prompt triggers an inflammatory syndrome with abnormal meta- 1234567890();,: 1234567890();,: diagnosis and immediate intervention, usually by emergency bolic pathways, including placental release of large amounts cesarean section, to save the fetus and reduce the risk of antiangiogenic factors such as soluble forms-like tyrosine of bleeding complications in the mother. The results from kinase-1 (sFlt-1). High plasma levels of these factors hinder Naruse et al. [1] highlight that this serious condition has the action of vascular endothelial growth factor (VEGF) and occurred after hospital admission in expectant patients with placental growth factor (PLGF) on receptors on the endo- early onset PE at a GA less than 34 weeks. -

Managing an Eclamptic Patient
BEST PRACTICES FOR DIAGNOSIS AND MANAGEMENT OF PREECLAMPSIA OBGMANAGEMENT Managing Baha M. Sibai, MD Professor and Chairman Department of Obstetrics an eclamptic patient and Gynecology, University of Cincinnati Most Ob/Gyns have little experience managing acute eclampsia, but all maternity units and obstetricians need to be prepared to diagnose and manage this grave threat. n eclamptic convulsion is frighten- with generalized muscular contractions. ing to behold. First, the woman’s In the second phase, which lasts A face becomes distorted, and her about a minute, the muscles of the body eyes protrude. Then her face acquires a alternately contract and relax in rapid congested expression, and foam may succession. This phase begins with the exude from her mouth. Breathing stops. muscles of the jaw and rapidly encom- Because eclampsia is so frightening, passes eyelids, other facial muscles, and the natural tendency is to try to stop a con- body. If the tongue is unprotected, the vulsion, but this is not the wisest strategy. woman often bites it. Rather, the foremost priorities are to Coma sometimes follows the convul- avoid maternal injury and support cardio- sion, and deep, rapid breathing usually IN THIS ARTICLE vascular functions. How to do this, and begins as soon as the convulsion stops. In how to prevent further convulsions, moni- fact, maintaining oxygenation typically is ❙ Signs and tor and deliver the fetus, and avert compli- not a problem after a single convulsion, symptoms cations are the focus of this article. and the risk of aspiration is low in a well Page 40 Since eclampsia may be fatal for both managed patient.