Seed Germination Is Recognized by the Emergence Seed Germination of the Radicle from the Seed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Physiology
PLANT PHYSIOLOGY Vince Ördög Created by XMLmind XSL-FO Converter. PLANT PHYSIOLOGY Vince Ördög Publication date 2011 Created by XMLmind XSL-FO Converter. Table of Contents Cover .................................................................................................................................................. v 1. Preface ............................................................................................................................................ 1 2. Water and nutrients in plant ............................................................................................................ 2 1. Water balance of plant .......................................................................................................... 2 1.1. Water potential ......................................................................................................... 3 1.2. Absorption by roots .................................................................................................. 6 1.3. Transport through the xylem .................................................................................... 8 1.4. Transpiration ............................................................................................................. 9 1.5. Plant water status .................................................................................................... 11 1.6. Influence of extreme water supply .......................................................................... 12 2. Nutrient supply of plant ..................................................................................................... -

What Are Soybeans?
candy, cakes, cheeses, peanut butter, animal feeds, candles, paint, body lotions, biodiesel, furniture soybeans USES: What are soybeans? Soybeans are small round seeds, each with a tiny hilum (small brown spot). They are made up of three basic parts. Each soybean has a seed coat (outside cover that protects the seed), VOCABULARY cotyledon (the first leaf or pair of leaves within the embryo that stores food), and the embryo (part of a seed that develops into Cultivar: a variety of plant that has been created or a new plant, including the stem, leaves and roots). Soybeans, selected intentionally and maintained through cultivation. like most legumes, perform nitrogen fixation. Modern soybean Embryo: part of a seed that develops into a new plant, cultivars generally reach a height of around 1 m (3.3 ft), and including the stem, leaves and roots. take 80–120 days from sowing to harvesting. Exports: products or items that the U.S. sells and sends to other countries. Exports include raw products like whole soybeans or processed products like soybean oil or Leaflets soybean meal. Fertilizer: any substance used to fertilize the soil, especially a commercial or chemical manure. Hilum: the scar on a seed marking the point of attachment to its seed vessel (the brown spot). Leaflets: sub-part of leaf blade. All but the first node of soybean plants produce leaves with three leaflets. Legume: plants that perform nitrogen fixation and whose fruit is a seed pod. Beans, peas, clover and alfalfa are all legumes. Nitrogen Fixation: the conversion of atmospheric nitrogen Leaf into a nitrogen compound by certain bacteria, such as Stem rhizobium in the root nodules of legumes. -

International Union for the Protection of New Varieties of Plants
E TG/81/7(proj.1) ORIGINAL: English DATE: 2018-04-05 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS Geneva DRAFT * COMMON SUNFLOWER UPOV Code(s): HLNTS_ANN Helianthus annuus L. GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY prepared by experts from Hungary to be considered by the Technical Working Party for Agricultural Crops at its forty-seventh session, to be held in Naivasha, Kenya, from 2018-05-21 to 2018-05-25 Disclaimer: this document does not represent UPOV policies or guidance Alternative names:* Botanical name English French German Spanish Helianthus annuus L. Common Sunflower Soleil, Tournesol Sonnenblume Girasol The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] TG/81/7(proj.1) Common Sunflower, 2018-04-05 2 TABLE OF CONTENTS PAGE 1. -

Evaluating Ethylene Sensitivity Using Mature Plant Screens and the Seedling Hypocotyl Response
Evaluating Ethylene Sensitivity Using Mature Plant Screens and the Seedling Hypocotyl Response THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Nichole Frances Edelman, B. A. Graduate Program in Horticulture and Crop Science The Ohio State University 2013 Master's Examination Committee: Dr. Michelle L. Jones, Advisor Dr. Pablo Jourdan Dr. Claudio Pasian Copyrighted by Nichole Frances Edelman 2013 Abstract Ethylene (C2H4) is a gaseous hormone produced by plants in response to environmental stress and during growth and development. Sensitive seedlings exposed to ethylene gas will exhibit an exaggerated apical hook, thickened hypocotyl, and reduced hypocotyl elongation. Collectively these symptoms are known as the triple response, and have been used as a screen to identify ethylene mutants. Exposure to ethylene gas at the mature plant stage can induce flower, bud, or leaf abscission, flower senescence, leaf chlorosis (yellowing), or leaf epinasty (downward curvature). Sensitivity can vary between species and by developmental stage or tissue (flower vs leaf). Ethylene can damage plants during production, shipping, and retailing. The objectives of this research were 1) to determine if the seedling hypocotyl elongation screen could be used to predict the sensitivity of mature plants at the marketable stage; 2) to identify ethylene sensitivity differences (levels of sensitivity and symptoms) between accessions within Solanaceae; and 3) to identify ethylene sensitivity differences at different developmental stages (seedling, juvenile, and mature plants). Seedlings were germinated on filter paper saturated with 1- aminocyclopropane-1-carboxylic acid (ACC) and sensitivity was determined based on the hypocotyl lengths relative to the control (0 µM ACC). -

Effects of Root Pruning on Germinated Pecan Seedlings
PROPAGATION AND TISSUE CULTURE HORTSCIENCE 50(10):1549–1552. 2015. if pruning the roots shortly after germination stimulates lateral root formation. Similarly, little work has been attempted to consider Effects of Root Pruning on Germinated the effects of taproot pruning in young pecan seedlings back to different lengths on root Pecan Seedlings regeneration. The objective of this study 1 was to determine if root pruning at different Rui Zhang, Fang-Ren Peng , Pan Yan, Fan Cao, periods after seed germination affects pecan and Zhuang-Zhuang Liu seedling root and shoot growth and to evaluate College of Forestry, Nanjing Forestry University, 159 Longpan Road, different degrees of taproot pruning on root Nanjing 210037, China initiation. Dong-Liang Le Materials and Methods College of Forestry, Nanjing Forestry University, 159 Longpan Road, Nanjing 210037, China; and Nanjing Green Universe Pecan Science and The test was established at Lvzhou Pecan Research Station, Nanjing, China (lat. Technology Co. Ltd., 38 Muxuyuan Street, Nanjing 210007, China 32.05°N, long. 118.77°E). Open-pollinated Peng-Peng Tan seeds of the Chinese selection ‘Shaoxing’ were used as seed stock to produce seedlings. College of Forestry, Nanjing Forestry University, 159 Longpan Road, ‘Shaoxing’ had a small nut of very good Nanjing 210037, China quality with 50% percentage fill. The seeds averaged 30.4 mm in length, 20.9 mm in Additional index words. Carya illinoinensis, taproot, lateral root, shoot growth width, and 5.4 g in weight. Seeds were Abstract. Root systems of pecan trees are usually dominated by a single taproot with few collected on 7 Nov. -
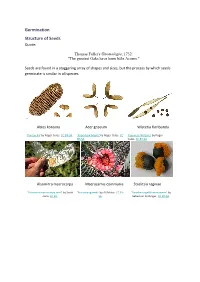
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -
4-H Growing Things Series Discovering the Science of Plants Welcome 4-H Leaders!
4-H Growing Things Series Discovering the Science of Plants Welcome 4-H Leaders! Welcome to the “Discovering the Science of Plants” project. There is lots of information, fun facts and hands on activities that cover the basic scientific Table of Contents principles of how plants grow and survive. This guide provides you with Introduction 1 project meeting plans (Skill Builders) that include, a skills list, background information, activity suggestions, and ways to know if your members have Project Summary 2 learned the skills identified. Skill Builder 1: 5 What is a plant? In this project, members will examine, by learning to do by doing, how plants grow and how they are used in everyday life. The Leader Guide is written with Skill Builder 2: 11 the expectation that the project leader(s) will have a working knowledge about Seeds for the the project topics and how they work. If not, you may need to do some Future pre-work / research on the activities, or recruit assistance for certain sections. Skill Builder 3: 18 Getting to the Be sure to try out activities, demonstrations or hands on work ahead of time to Root of it ensure you have an understanding of each Skill Builder - this also allows for any adjustments should an activity not work for you or if any equipment or supplies Skill Builder 4: 26 are unavailable. Leaves and Stems: the Power The 3D’s of Learning - Each Skill Builder has three sections of learning called Generators “Dream it!”, “Do it!” and “Dig it!”. Below is a description of each. -

Soybean Growth and Development & Management Information Fo Replant Decisions
MAGRCORE Metadata, citation and similar papers at core.ac.uk GOVSProvided by University of Minnesota Digital Conservancy MN 2500 AGFO-5701 MINNESOTA EXTENSION SERVICE I:,:, I UNIVERSITY OF MINNESOTA AGRICULTURE Soybean Growth and Development & Management Information fo UNIVf:R&TYoF MINNESOTA J DOCUMENTS ~ Replant Decisions JUL ·2s 1991 ,1 ·I shoot apex emerging decomposing radicle · - seed coat primary root L.L. Hardman J.L. Gunsolus Extension Agronomist-Crops Extension Agronomist-Weed Control Dept. of Agronomy & Dept. of Agronomy & Plant Genetics Plant Genetics Univ. of MN, St. Paul Univ. of MN, St. Paul SOYBEAN GROWTH AND DEVELOPMENT stored food is removed the cotyledons tum yellow and shrivel before dropping off the plant. Loss of one or more of the cotyledons before the food reserves are fully utilized can slow An understanding of how a soybean plant develops can help you early plant growth or result in death of the plant if photosynthetic make important management decisions. This section discusses leaf tissue is not formed quickly. various seed and plant parts, explains how the soybean plant develops utilizing standardized vegetative and reproductive stage descriptions which are used by the National Crop Insurance Services (NCIS), and describes some of the important factors which affect growth and development of the soybean plant. A soybean seed consists of a miniature plant attached to two nutrient storage reservoirs (cotyledons or seed halves) surrounded by a protective outer wrapper or seed coat called a testa (Figure 1). The cotyledons contain about 40% protein, 20% oil, and about 35% carbohydrate. These materials provide nutrients to the developing embryo during the germination process. -

Osbhlh073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice
plants Article OsbHLH073 Negatively Regulates Internode Elongation and Plant Height by Modulating GA Homeostasis in Rice 1, 2, 3 4 2 Jinwon Lee y, Sunok Moon y, Seonghoe Jang , Sichul Lee , Gynheung An , Ki-Hong Jung 2,* and Soon Ki Park 1,* 1 School of Applied Biosciences, Kyungpook National University, Daegu 41566, Korea; [email protected] 2 Graduate School of Biotechnology & Crop Biotech Institute, Kyung Hee University, Yongin 17104, Korea; [email protected] (S.M.); [email protected] (G.A.) 3 World Vegetable Center Korea Office (W.K.O), Jellabuk-do 55365, Korea; [email protected] 4 Center for Plant Aging Research, Institute for Basic Science (IBS), Daegu 42988, Korea; [email protected] * Correspondence: [email protected] (K.-H.J.); [email protected] (S.K.P.) These authors contributed equally to this work: J.L., S.M. y Received: 5 March 2020; Accepted: 21 April 2020; Published: 23 April 2020 Abstract: Internode elongation is one of the key agronomic traits determining a plant’s height and biomass. However, our understanding of the molecular mechanisms controlling internode elongation is still limited in crop plant species. Here, we report the functional identification of an atypical basic helix-loop-helix transcription factor (OsbHLH073) through gain-of-function studies using overexpression (OsbHLH073-OX) and activation tagging (osbhlh073-D) lines of rice. The expression of OsbHLH073 was significantly increased in the osbhlh073-D line. The phenotype of osbhlh073-D showed semi-dwarfism due to deficient elongation of the first internode and poor panicle exsertion. Transgenic lines overexpressing OsbHLH073 confirmed the phenotype of the osbhlh073-D line. -

Tulipa Kaufmanniana Regel.) Propagation Under Ex Situ Conditions ISSN 2447-536X |
THE APPLICATION OF DIFFERENT REPRODUCTION TECHNIQUES FOR RARE SPECIES WATERLILY TULIP 450 Ornamental (HorticultureTULIPA KAUFMANNIANA REGEL.) PROPAGATION UNDER EX SITU CONDITIONS ISSN 2447-536X | HTTPS://ORNAMENTALHORTICULTURE.EMNUVENS.COM.BR/RBHO SCIENTIFIC ARTICLE The application of different reproduction techniques for rare species waterlily tulip (Tulipa kaufmanniana Regel.) propagation under ex situ conditions Nabieva Alexandra Yurievna1*, Gerasimovich Lyudmila Vladimirovna2 Abstract T. kaufmanniana is a threatened and endemic species of the Tien Shan Mountains with a complex of valuable ornamental features. However, the commercial usage of the plant is limited due to the restricted availability of the bulbs in nature, difficulties in overcoming seed dormancy and low efficiency of the species reproduction. Results of the investigation of the reproductive biology of this species in the culture conditions allowed us to characterize T. kaufmanniana as viable with successful seedage. In the work reported here the effect of low temperature on the proper development of embryos is addressed, and an attempt is made to clarify the effect of cytokinins (TDZ, BAP) and auxin (NAA) on the regeneration capacity of isolated T. kaufmanniana embryos for the adventitious shoots and bulblets formation. Direct shoot organogenesis was induced by the combined action of chilling and BAP or TDZ treatment. Among the variants tested, TDZ promoted the formation of adventitious bulbs in 48 % of the chilled embryos, compared to non-chilled embryos (0.0%) and embryos exposed to the same concentration of BAP (39%). The best culture condition for T. kaufmanniana embryos growth and bulblets formation consisted of chilling for 10 wk in the dark at 4 °C, 4 wk at 20 °C under 16 h light photoperiod and 10 wk at 4 °C in the dark. -

Difference Between Radicle and Plumule Key Difference
Difference Between Radicle and Plumule www.differencebetween.com Key Difference - Radicle vs Plumule All seeds contain embryos. Seed embryo creates a new plant after germination. Therefore the embryo is protected inside the seed by a hard covering called testa. The seed begins to grow when the right conditions are found such as moisture, warmth, sunlight, nutrient-rich soil etc. There are two main components of a plant; shoot and root. Stem, leaves and roots develop from different parts of the seed embryo. The radicle is the first part that emerges from the seed during the germination through the micropyle (seed pore). It makes the roots of the new plant. Plumule emerges after the radicle and makes the stem of the new seedling. Cotyledons form the first leaves of the seedling. The key difference between radicle and plumule is that radicle is the root forming part of the seed embryo while plume is the stem forming part of the seed embryo. The cotyledons of the seed embryo hold the radicle and the plume. What is Radicle? The radicle is the embryonic root of the plant. It is a part of the seedling that comes first from the seed during the germination. It comes out from the seed through the micropyle. Radicle grows downwards into the soil. A root cap protects the tip of the radicle. It absorbs water and nutrients and supplies to the leaves for starting photosynthesis. Embryonic stem or the hypocotyl is found above the radicle. Figure 01: Radicle Radicle emerges from the seed as a short white structure. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil.