Swain 13 (12Mnth).Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Section 5.3 Designations
Section 5.3 Designations Submitter Name/ Plan Provision Summary of Submission Decision Requested Submission No. and Point General Invercargill City Designation - New A designation is required to replace the designation known as D3 in the first generation District Plan. There exists a standpipe That the SDC grant a designation as shown on the map attached to Council structure already and there are plans for future development. The inclusion of a new designation would correct the original the submission, labelled “Designation on part section 11 Invercargill 224.1 mistake and recognise there is already a structure in existence and provide for future development for the ICC waterworks. (Certificate of title SL 145/298)” for the purpose “To allow development of air elimination and surge suppression on the Branxholme water supply pipelines”. PowerNet General The Whitehill Substation has not been included within the District Plan Designation schedule. The Notice of Requirement for a Include the White Hill Substation within the Designation Schedule. 241.12 designation was confirmed by Southland District Council on 5 October 2011. Requiring Authority: The Power Company Limited Purpose: White Hill Substation - Electricity Zone Substation and Ancillary Purposes. Location: Felton Road, Mossburn Legal Description: Part of Lot 1, DP338582 Radio New General Support in part. Would be useful to explain that written consent must be obtained from a requiring authority before a third party Amendment to section as below: Zealand may do anything that would prevent or hinder the public work “A designation is a provision made in the District Plan to give effect 100.1 to a requirement made by a requiring authority. -

Proposed Southland Water and Land Plan Part A
Proposed Southland Water and Land Plan Part A 29 January 2018 (Recommendations Report version – tracked changes) Mai ea i te pōo i te ti Mātānga. Mai ea ki ngāa hekenga kia Māaku. Otirāa, ka kii a ngā puna roi Mātā roimata a Rangi, ko tōona aroha kia Papatūānuku, kia kii ōona puna hei oranga mōona me ōona Taonga e noho ake nei. Ko tāatou, ngā kaitiaki o tēenei taonga tuku iho kia kaha i roto i te tapu, kia whai māana i roto i tōona wehi, kia u tōona wairua, ka whakanoa i muri ake nei. From the void, through the regions of the night, through the steps of the evolution, eventually arriving at the dampness, indeed filling the pools of Rangi which overflow eventually as tears of love on Papatūānuku. In turn her bosom is filled with those tears and she disperses them evenly to everything that grows on her. We Taāngata whenua and Te Taiao Tonga have the responsibility as protectors for this treasure handed down for use in its natural state with prestige, retaining its spiritual wellbeing so that we can continue to use it safely and wisely into the future. Proposed Southland Water and Land Plan (Recommendations Report, 29 January 2018) Page 2 Contents Part A Preamble ..................................................................................................................... 5 Te Mana o te Wai ........................................................................................................ 5 Introduction ................................................................................................................ 7 Purpose of -

Southland Conservancy
A Directory of Wetlands in New Zealand SOUTHLAND CONSERVANCY Te Anau Basin Wetland Complex (71) Location: 45o27'S, 167o46'E. To the east and southeast of Lake Te Anau, Southland, South Island. Area: c.2,400 ha. Altitude: 180-360 m. Overview: The Te Anau Basin Wetland Complex consists of seven distinct and isolated wetlands within the Te Anau Basin. These sites are the Dome Mire and Dismal Swamp area, Kepler Mire, Amoeboid Mire, Kakapo Swamp, Dunton Bog and two areas within the Snowdon Forest. The Dome Mire and Dismal Swamp area and Kepler Mire are described in greater detail as Sites 71a and 71b, respectively. All of the wetlands have a similar glacial origin; however, individual sites vary as a consequence of their history, drainage (water table, amounts of ponded water), fertility, topography etc. The complex of peatlands contains a rich variety of plant communities which include several significant plant distributions and provide important habitat for wildlife. Physical features: The Te Anau Basin lies on the eastern margin of Fiordland, a gneiss/schist/granite massif uplifted by the Alpine Fault on its western margin and carved by extensive glaciation through the Quaternary. On its eastern flank, down-faulting in the Te Anau Basin area has contributed to the preservation of soft Tertiary sedimentary rocks, and the deposition of glacial gravels. During the last glaciation, glaciers occupied much of the basin. Depressions and areas of limited drainage developed in the moraines, tills and outwash gravels as the glaciers retreated. Wetlands have developed on these glacial outwash deposits of last glacial to post-glacial age. -

40 Wetlands to Visit
! Wetlands to visit in the South Island 22. Farewell Spit (Onetahua), 23. Mangarakau Swamp, Westhaven and Stewart Island/Rakiura Golden Bay (Whanganui Inlet) New Zealand’s longest sandspit system, extending eastward A large, relatively pristine freshwater swamp at the southern into the Tasman Sea for approximately 30 km, shelters tidal end of the Whanganui Inlet, south of Farewell Spit, on the 2!2 mudflats exposed at low tide for up to 6 km seaward. These west coast. This is a magical location—to the west are tidal 2!3 ! 2!4 mudflats and the dunes provide a haven for over 90 bird bays and inlets and towering limestone bluffs, and to the east Nelson species including black swan, Australasian gannet, Caspian the forested ranges of Kahurangi National Park. Westhaven ! tern/tarānui, southern black-backed gull/karoro, bar-tailed (Whanganui Inlet) is one of the largest and least modified godwit/kuaka, red knot and variable oystercatcher/tōrea. Many estuaries in New Zealand and was formally protected in 1994 of these birds migrate thousands of kilometres each year from with the creation of the Westhaven (Te Tai Tapu) Marine the northern hemisphere. Reserve and the Westhaven (Whanganui Inlet) Wildlife The spit forms a Ramsar wetland of international importance Management Reserve. and it is also an East Asian-Australasian Flyway Network site Places to explore for migratory birds. The area has been a flora and fauna reserve It is worth starting your visit at the Friends of Mangarakau and wildlife sanctuary since 1938. Visitor Centre and doing the short walks to the lookouts and Hokitika the hidden jewel that is Lake Mangarakau. -

Before the Hearing Panel Invercargill / Waihōpai
BEFORE THE HEARING PANEL INVERCARGILL / WAIHŌPAI UNDER the Resource Management Act 1991 IN THE MATTER of the proposed Southland Water and Land Plan MEMORANDUM OF COUNSEL FOR THE SOUTHLAND REGIONAL COUNCIL 26 September 2017 WYNN WILLIAMS Southland Regional Council's LAWYERS Solicitor CHRISTCHURCH Level 5, Wynn Williams House, 47 Hereford Street, Solicitor: P A C Maw P O Box 4341, DX WX11179, ([email protected]) CHRISTCHURCH 8140 Tel 0064 3 3797622 Fax 0064 3 3792467 1 MAY IT PLEASE THE PANEL 1 The purpose of this Memorandum is to update the Hearing Panel on the status of the proposed Southland Regional Policy Statement 2012 (pRPS). 2 At the time of preparing the Section 42A Report, both the pRPS and the operative Southland Regional Policy Statement 1997 (RPS 1997) were relevant to the decisions on submissions on the proposed Southland Water and Land Plan (pSWLP). 3 In the Section 42A Report, the Council Officers advised the Panel that:1 (a) the pSWLP must give effect to the RPS 1997; (b) the Panel is required to have regard to the pRPS, although it was submitted that the pSWLP should give effect to the parts of the pRPS that are 'beyond challenge'; and 4 The Council Officers considered that the pSWLP gives effect to the RPS and pRPS.2 5 At the time of preparing the Section 42A Report, the provisions of the pRPS that were under challenge were in Chapter 4 (Water) and Chapter 7 (Coast), as follows:3 (a) Objective WQUAL.2 - Lowland water bodies; (b) Policy WQUAL.3 - Wetlands and outstanding freshwater bodies; (c) Notified Policy WQUAL.4 - Waituna Lagoon; (d) Notified Method WQUAL.13 - Waituna Catchment. -

Nzbotsoc No 64 June 2001
NEW ZEALAND BOTANICAL SOCIETY NEWSLETTER NUMBER 64 JUNE 2001 New Zealand Botanical Society President: Jessica Beever Secretary/Treasurer: Anthony Wright Committee: Bruce Clarkson, Colin Webb, Carol West Address: c/- Canterbury Museum Rolleston Avenue CHRISTCHURCH 8001 Subscriptions The 2001 ordinary and institutional subs are $18 (reduced to $15 if paid by the due date on the subscription invoice). The 2001 student sub, available to full-time students, is $9 (reduced to $7 if paid by the due date on the subscription invoice). Back issues of the Newsletter are available at $2.50 each from Number 1 (August 1985) to Number 46 (December 1996), $3.00 each from Number 47 (March 1997) to Number 50 (December 1997), and $3.75 each from Number 51 (March 1998) onwards. Since 1986 the Newsletter has appeared quarterly in March, June, September and December. New Subscriptions are always welcome and these, together with back issue orders, should be sent to the Secretary/Treasurer (address above). Subscriptions are due by 28th February each year for that calendar year. Existing subscribers are sent an invoice with the December Newsletter for the next year's subscription which offers a reduction if this is paid by the due date. If you are in arrears with your subscription a reminder notice comes attached to each issue of the Newsletter. Deadline for next issue The deadline for the September 2001 issue (Number 65) is 25 August 2001. Please post contributions to: Joy Talbot 23 Salmond Street Christchurch 8002 Send contributions by e-mail to [email protected]. Files can be in WordPerfect (version 8 or earlier), MS Word (version 6 or earlier) or saved as RTF or ASCII. -

Proposed Southland Water and Land Plan: Part A
Proposed Southland Water and Land Plan: Part A Version showing recommendations from the Hearing Report prepared under Section 42A of the Resource Management Act 1991 26 May 2017 How to read this document This version of the Plan does not have any legal weight. It has been prepared to help the Hearing Panel, Council staff, and submitters understand what changes have been recommended to the provisions in the Plan since it was notified. Key Appearance Explanation Black text Parts of the Proposed Southland Water and Land Plan as they were when notified on 3 June 2016. Black text with underlining Additions recommended by Council staff through the Section 42A Report. Black text with strikethrough Deletions recommended by Council staff through the Section 42A Report. Red text with underlining Further additions recommended by Council staff in response to questions from the Hearing Panel. Red text with strikethrough Further deletions recommended by Council staff in response to questions from the Hearing Panel. Proposed Southland Water and Land Plan Page 2 Mai ea i te po i te tiMātānga. Mai ea ki nga hekenga kia Maku. Otira, ka kii a ngā puna roi Mātā a Rangi, ko tona aroha kia Papatūānuku, kia kii ona puna hei oranga mona me ona Taonga e noho ake nei. Ko tatou, ngā kaitiaki o tenei taonga tuku iho kia kaha i roto i te tapu, kia whai mana i roto i tona wehi, kia u tona wairua, ka whakanoa i muri ake nei. From the void, through the regions of the night, through the steps of the evolution, eventually arriving at the dampness, indeed filling the pools of Rangi which overflow eventually as tears of love on Papatūānuku. -

IN the MATTER of the Resource Management Act 1991
IN THE MATTER of the Resource Management Act 1991 AND IN THE MATTER of Applications for Resource Consent to Discharge Treated Wastewater to land and Air – APP-302625-01 AND IN THE MATTER of a Notice of Requirement to Designate Land for the Te Anau Manapouri Wastewater Discharge Area BY SOUTHLAND DISTRICT COUNCIL DECISION ON RESOURCE CONSENT APPLICATIONS AND REPORT AND RECOMMENDATION ON NOTICE OF REQUIREMENT Hearing Panel Mr Denis Nugent, Chair Ms Yvette Couch-Lewis Mr Rob Potts The hearing panel has been delegated full decision-making powers by Environment Southland in respect of the application APP-302625-01 and is to make a recommendation to Southland District Council on the Notice of Requirement (“NoR”). Summary of Decisions & Recommendations Decision on Resource Consent Applications A. Consent is granted to Southland District Council as follows: (a) Discharge Permit No. 302625: To discharge treated wastewater onto land from the Te Anau wastewater treatment plant, via a spray irrigation system, onto land known as the Kepler Block legally ii described as Lot 2 DP 410687 subject to the conditions set out in Part A of Appendix 4; and (b) Discharge Permit to Air No. 302625A: To discharge contaminants to air, namely odour and spray drift, arising from the irrigation of treated wastewater authorised by Discharge Permit 302625, subject to the conditions set out in Part B of Appendix 4. Recommendations to Southland District Council on Notice of Requirement B. We recommend that the Southland District Council confirm the Notice of Requirement for Designation D80 by the Water and Waste Services Division of the Council to designate land for public utility purposes on Lot 2 DP 410687, subject to the modifications and conditions set out below and as shown on Figure 1 in Appendix 5. -
Southland Protection Strategy
SOUTHLAND PROTECTION STRATEGY A REPORT TO THE NATURE HERITAGE FUND COMMITTEE M.A. Harding Southland Protection Strategy Published by the Nature Heritage Fund PO Box 10-420 Wellington May 1999 ISBN CONTENTS 1.0 Introduction .............................................................................................. 1 2.0 Methodology ............................................................................................. 2 2.1 Describing the indigenous ecosystems in Southland .................... 3 2.2 Estimating the original and present extent of ecosystems ............ 5 2.3 Estimating the adequacy of protection .......................................... 6 3.0 Indigenous ecosystems in Southland ...................................................... 7 4.0 Analysis of the indigenous ecosystems by ecological district ............... 9 Aspiring Ecological Region Dart ED (51.07) .......................................................................... 11 Central Otago Ecological Region Old Man ED (67.05) ................................................................... 12 Lammerlaw Ecological Region Tapanui ED (68.03) .................................................................... 14 Catlins Ecological Region Waipahi ED (70.01) .................................................................... 16 Tahakopa ED (70.02) .................................................................. 19 Olivine Ecological Region Pyke ED (71.02).......................................................................... 23 Fiord Ecological Region -
Lakes Manapouri and Teanau
BURROWS AND DansoN: MIRES OF MANAPOURI-'- TE ANAU LOWLANDS 75 LAKES MANAPOURI AND TE ANAU MIRES OF THE MANAPOURI- TE AN AU LOWLANDS C. J. BURROWS AND A. T. DOBSON Department of Botany, University of Canterbury SUMMARY: This is a preliminary account of the wetlands near Lakes Manapouri and Te Anau, New Zealand. It discusses briefly the vegetational history of the area and describes the general nature and vegetation of certain of the mires. TERl\UNOLOGY stratigraphy of deposits of a mire, to distinguish between those elements ,vhich have been derived We wi!)h to avoid using terms which might in situ and those which have been derived else- be considered colloquial and imprecise or which where (see e.g. West 1968). have been given precise and fairly limiting defini- tions e]sewhere. Therefore, throughout this paper, THE LOCALITY AND HISTORY OF THE 'VETLANDS the terms 'wetland' and more especiaHy 'mire' are emp]oyed. "Vetland' is a general term for Lakes ~fanapouri and Te Anau lie on the east- an ecosystem which has a water-table above, at, ern margin of the hard rock highland of Fiord- or just below the substrate surlace so that the ]and, their basins partially surrounded by steep substrate is water-saturated for most of the year. mountainsides cut into PalGeozoie gneisses and ']\'Iire' is 'also a broad term and is used when granites. The lakes drain by way of the 'Vaiau referring to specific wetland sites carrying any River into the Te Anau basin, a depression con- type of macrophytic vegetation. It has had wide- taining soft sediments of Tertiary and Quaternary spread use elsewhere, particularly by Scandina- age (Wood 1962, 1966). -
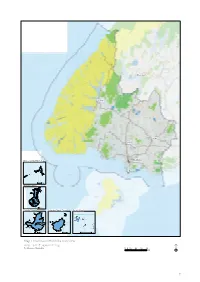
Draft Southland CMS 2013 Vol II Maps 1 to 4
Queenstown Te Anau GORE Winton INVERCARGILL Snares Islands/Tini Heke Bluff 1 km Auckland Islands 1 km Campbell Islands Antipodes Island Group Bounty Islands 1 km 1 km 1 km Map 1 Southland Murihiku overview Conservation Management Strategy Southland Murihiku 05 10 20 30 40 Km 7 Big Bay Milford Sound/ Piopiotahi Pyke River Hollyford Track Upper Milford Track Hollyford Routeburn Track Arthur Milford Road Journey Key Summit Clinton Greenstone/ Caples Track Eglinton - upper Inset Queenstown Eglinton Mararoa Kepler Track - lower River Irene-Windward Western Murchison Mountains Mavora Lakes Secretary Island Mount Eastern Garvie Forbes Murchison Mountains Mararoa River Mountains Te Anau scrub Eyre Mountains Wilmott Waikaia beech forests Croydon Bush Resolution Island Upper Grebe Oreti River bed Takitimu Mountains Anchor & Indian Islands Dean Burn-Mangapiri Blue Mountains wetland West Cape Lake Lonekers Hauroko Lill Burn Bush wetlands Hokonui GORE Lake Hills Poteriteri Bayswater Peatland Scenic Reserve Winton Chalky Spit Islands Island Forest Hill Scenic Reserve Longwood Waitutu Marshall Bush Range Scenic Reserve Wyndham Southern Fiordland Coast Scenic Reserve Snares Islands /Tini Heke Lake George /Urewera INVERCARGILL Otatara-Bushy Point New River Estuary Haldane Bluff Bay Solander Waituna-Awarua Islands Plains Forest Hill Lake Brunton and dunes 0 5 km The Bluff/ Motupōhue Auckland Islands Curio Bay/ Porpoise Bay 0 30 km Campbell Islands Antipodes Island Group Bounty Islands 0 10 km 0 5 km 0 2 km Map 2 Indicative ecosystem and destination priorities -

SAR 04-83-03-83 DOCDM-2845010 29 July 2016 Southland Water And
SAR 04-83-03-83 DOCDM-2845010 29 July 2016 Southland Water and Land Plan Southland Regional Council Private Bag 90116 INVERCARGILL 9840 Attention: Anita Dawe Dear Anita, PROPOSED SOUTHLAND WATER AND LAND PLAN 3 June 2016 Please find enclosed the submission by the Director-General of Conservation in respect of the proposed Southland Land and Water Plan 2016. The submission identifies the Director- General’s concerns. Please contact Ken Murray in the first instance if you wish to discuss any of the matters raised in this submission (03 3713759 [email protected]). Yours sincerely Tony Preston Operations Manager Murihiku Department of Conservation Te Papa Atawhai DOC-2845010 Christchurch Shared Services Private Bag 4715, Christchurch Mail Centre, Christchurch 8140, New Zealand www.doc.govt.nz RESOURCE MANAGEMENT ACT 1991 SUBMISSION ON PROPOSED SOUTHLAND WATER AND LAND PLAN 2016 TO: Southland Regional Council SUBMISSION ON: Proposed Southland water and Land Plan 2016 NAME: Director-General of Conservation ADDRESS: RMA Shared Services Department of Conservation Private Bag 4715 Christchurch Mail Centre 8140 Attn: Ken Murray STATEMENT OF SUBMISSION BY THE DIRECTOR-GENERAL OF THE DEPARTMENT OF CONSERVATION Pursuant to clause 6 of the First Schedule of the Resource Management Act 1991 (RMA), I, Tony Preston, Operations Manager Murihiku, Department of Conservation acting upon delegation from the Director-General of the Department of Conservation, make the following submission in respect of the Proposed Southland Water and Land Plan to the Southland Regional Council. 1. This is a submission on the Proposed Southland Water and Land Plan. 2. The specific provisions of the Proposed Plan that my submission relates to are set out in Attachments 1 to this submission.