Effects of an Alien Vertebrate Herbivore on the Regeneration of Endangered
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bruxner Park Flora Reserve Working Plan
Bruxner Park Flora Reserve Working Plan Working Plan for Bruxner Park Flora Reserve No 3 Upper North East Forest Agreement Region North East Region Contents Page 1. DETAILS OF THE RESERVE 2 1.1 Introduction 2 1.2 Location 2 1.3 Key Attributes of the Reserve 2 1.4 General Description 2 1.5 History 6 1.6 Current Usage 8 2. SYSTEM OF MANAGEMENT 9 2.1 Objectives of Management 9 2.2 Management Strategies 9 2.3 Management Responsibility 11 2.4 Monitoring, Reporting and Review 11 3. LIST OF APPENDICES 11 Appendix 1 Map 1 Locality Appendix 1 Map 2 Cadastral Boundaries, Forest Types and Streams Appendix 1 Map 3 Vegetation Growth Stages Appendix 1 Map 4 Existing Occupation Permits and Recreation Facilities Appendix 2 Flora Species known to occur in the Reserve Appendix 3 Fauna records within the Reserve Y:\Tourism and Partnerships\Recreation Areas\Orara East SF\Bruxner Flora Reserve\FlRWP_Bruxner.docx 1 Bruxner Park Flora Reserve Working Plan 1. Details of the Reserve 1.1 Introduction This plan has been prepared as a supplementary plan under the Nature Conservation Strategy of the Upper North East Ecologically Sustainable Forest Management (ESFM) Plan. It is prepared in accordance with the terms of section 25A (5) of the Forestry Act 1916 with the objective to provide for the future management of that part of Orara East State Forest No 536 set aside as Bruxner Park Flora Reserve No 3. The plan was approved by the Minister for Forests on 16.5.2011 and will be reviewed in 2021. -

Regional Landscape Surveillance for New Weed Threats Project 2016-2017
State Herbarium of South Australia Botanic Gardens and State Herbarium Economic & Sustainable Development Group Department of Environment, Water and Natural Resources Milestone Report Regional Landscape Surveillance for New Weed Threats Project 2016-2017 Milestone: Annual report on new plant naturalisations in South Australia Chris J. Brodie, Jürgen Kellermann, Peter J. Lang & Michelle Waycott June 2017 Contents Summary .................................................................................................................................... 3 1. Activities and outcomes for 2016/2017 financial year .......................................................... 3 Funding .................................................................................................................................. 3 Activities ................................................................................................................................ 4 Outcomes and progress of weeds monitoring ........................................................................ 6 2. New naturalised or questionably naturalised records of plants in South Australia. .............. 7 3. Description of newly recognised weeds in South Australia .................................................. 9 4. Updates to weed distributions in South Australia, weed status and name changes ............. 23 References ................................................................................................................................ 28 Appendix 1: Activities of the -
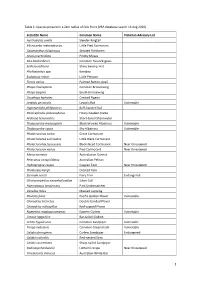
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

(Lamiaceae and Verbenaceae) Using Two DNA Barcode Markers
J Biosci (2020)45:96 Ó Indian Academy of Sciences DOI: 10.1007/s12038-020-00061-2 (0123456789().,-volV)(0123456789().,-volV) Re-evaluation of the phylogenetic relationships and species delimitation of two closely related families (Lamiaceae and Verbenaceae) using two DNA barcode markers 1 2 3 OOOYEBANJI *, E C CHUKWUMA ,KABOLARINWA , 4 5 6 OIADEJOBI ,SBADEYEMI and A O AYOOLA 1Department of Botany, University of Lagos, Akoka, Yaba, Lagos, Nigeria 2Forest Herbarium Ibadan (FHI), Forestry Research Institute of Nigeria, Ibadan, Nigeria 3Department of Education Science (Biology Unit), Distance Learning Institute, University of Lagos, Akoka, Lagos, Nigeria 4Landmark University, Omu-Aran, Kwara State, Nigeria 5Ethnobotany Unit, Department of Plant Biology, Faculty of Life Sciences, University of Ilorin, Ilorin, Nigeria 6Department of Ecotourism and Wildlife Management, Federal University of Technology, Akure, Ondo State, Nigeria *Corresponding author (Email, [email protected]) MS received 21 September 2019; accepted 27 May 2020 The families Lamiaceae and Verbenaceae comprise several closely related species that possess high mor- phological synapomorphic traits. Hence, there is a tendency of species misidentification using only the mor- phological characters. Herein, we evaluated the discriminatory power of the universal DNA barcodes (matK and rbcL) for 53 species spanning the two families. Using these markers, we inferred phylogenetic relation- ships and conducted species delimitation analysis using four delimitation methods: Automated Barcode Gap Discovery (ABGD), TaxonDNA, Bayesian Poisson Tree Processes (bPTP) and General Mixed Yule Coalescent (GMYC). The phylogenetic reconstruction based on the matK gene resolved the relationships between the families and further suggested the expansion of the Lamiaceae to include some core Verbanaceae genus, e.g., Gmelina. -

Invasion and Management of a Woody Plant, Lantana Camara L., Alters Vegetation Diversity Within Wet Sclerophyll Forest in Southeastern Australia
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Ben Gooden University of Wollongong, [email protected] Kris French University of Wollongong, [email protected] Peter J. Turner Department of Environment and Climate Change, NSW Follow this and additional works at: https://ro.uow.edu.au/scipapers Part of the Life Sciences Commons, Physical Sciences and Mathematics Commons, and the Social and Behavioral Sciences Commons Recommended Citation Gooden, Ben; French, Kris; and Turner, Peter J.: Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia 2009. https://ro.uow.edu.au/scipapers/4953 Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Invasion and management of a woody plant, Lantana camara L., alters vegetation diversity within wet sclerophyll forest in southeastern Australia Abstract Plant invasions of natural communities are commonly associated with reduced species diversity and altered ecosystem structure and function. This study investigated the effects of invasion and management of the woody shrub Lantana camara (lantana) in wet sclerophyll forest on the south-east coast of Australia. The effects of L. camara invasion and management on resident vegetation diversity and recruitment were determined as well as if invader management initiated community recovery. Vascular plant species richness, abundance and composition were surveyed and compared across L. -

Vegetation and Flora of Booti Booti National Park and Yahoo Nature Reserve, Lower North Coast of New South Wales
645 Vegetation and flora of Booti Booti National Park and Yahoo Nature Reserve, lower North Coast of New South Wales. S.J. Griffith, R. Wilson and K. Maryott-Brown Griffith, S.J.1, Wilson, R.2 and Maryott-Brown, K.3 (1Division of Botany, School of Rural Science and Natural Resources, University of New England, Armidale NSW 2351; 216 Bourne Gardens, Bourne Street, Cook ACT 2614; 3Paynes Lane, Upper Lansdowne NSW 2430) 2000. Vegetation and flora of Booti Booti National Park and Yahoo Nature Reserve, lower North Coast of New South Wales. Cunninghamia 6(3): 645–715. The vegetation of Booti Booti National Park and Yahoo Nature Reserve on the lower North Coast of New South Wales has been classified and mapped from aerial photography at a scale of 1: 25 000. The plant communities so identified are described in terms of their composition and distribution within Booti Booti NP and Yahoo NR. The plant communities are also discussed in terms of their distribution elsewhere in south-eastern Australia, with particular emphasis given to the NSW North Coast where compatible vegetation mapping has been undertaken in many additional areas. Floristic relationships are also examined by numerical analysis of full-floristics and foliage cover data for 48 sites. A comprehensive list of vascular plant taxa is presented, and significant taxa are discussed. Management issues relating to the vegetation of the reserves are outlined. Introduction The study area Booti Booti National Park (1586 ha) and Yahoo Nature Reserve (48 ha) are situated on the lower North Coast of New South Wales (32°15'S 152°32'E), immediately south of Forster in the Great Lakes local government area (Fig. -

Wells Crossing to Iluka Road
Upgrading the Pacific Highway Warrell Creek to Urunga Environmental assessment Volume 2 – Working paper 1 Flora and fauna January 2010 Working paper 1 –Flora and fauna assessment 7. References Adam, P. 1995, Urbanisation and Transport. Pp. 55 - 75 in Conserving Biodiversity: Threats and Solutions, Ed. Bradstock, R. A., Auld, T. D., Keith, D. A., Kingsford, R. T., Lunney, D. & Siversten, D. P. Surrey Beatty and Sons & NPWS, Chipping Norton. Allison, F. R. & Hoye, G. A. 1995, Eastern Little Freetail Bat. In The Mammals of Australia (ed. by Strahan, R.). Australian Museum/ Reed Publications, Sydney. Anderson, J. R. 1993, ‘State of the Rivers’ Project – Report 2. Implementation Manual, A Report to Department of Primary Industries, Queensland. Andrews, A. 1990, Fragmentation of habitat by roads and utility corridors: a review in Aust. Zool. 26, pp 130 - 141. ANPC (2004). Guidelines for the Translocation of Threatened Plants in Australia. 2nd Edition. Australian Network for Plant Conservation. ANSWW 2007. Atlas of NSW Wildlife: Department of Environment and Conservation (NPWS). ANZECC/ARMCANZ (2000), ‘Australian and New Zealand Guidelines for Fresh and Marine Water Quality’. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand. Ausroads (2001). Road Runoff and Drainage: Environmental Impacts and Management Options. Auld, B.A. & Medd, R.W. 1987, Weeds - an illustrated botanical guide to the weeds of Australia, Inkata Press: Sydney. Bali, R. (2005). Discussion Paper – Compensating for Edge Effects. Unpublished report prepared for the Roads and Traffic Authority, Sydney. Benwell, A. S. (2003). Yelgun to Chinderah Highway Upgrade Three Year Monitoring Report on Translocations of Threatened and Rare Rainforest Plant Species. -

Antarctic Beech (Nothofagus Moorei)
Dandarrga Nursery Native Species Labels A - M Antarctic Beech (Nothofagus moorei) Nothofagaceae A Gondwana rainforest tree 25 – 50 m Flowers Nov - Dec, seed pods Dec - Feb Range: High altitude rainforest of eastern Australia. This tree can reach a great age. New growth is red, and the complex root structure can grow multiple trunks, adorned with epiphytic orchids, ferns, fungi, mosses, liverworts and lichens. Bamboo Grass (Austrostipa ramosissima) Poaceae Native grass up to 1 to 2.5 m tall, 1.5 m wide Flowers: year round Range: S.E NSW to N.E QLD Stout Bamboo Grass is a tall ornamental grass. Fast growing and long lived. Useful container or border plant or for erosion and weed control. Attracts birds and small reptiles. Hardy; frost, drought and damp tolerant and grows in most soil conditions. Can be cut back hard to rejuvenate. Grows best with full or partial sun in shelter. Banana Bush (Tabernaemontana pandacaqui) Apocynaceae Deciduous shrub or small tree 1.5-14m Flowers: White; spring/summer Range: Manning River NSW to Cooktown QLD Normally growing to 1.5-3m in cultivation and can be pruned. Dense understory shrub with pretty tubular scented flowers. Unusual orange/ yellow fruit resemble small bananas but are poisonous to eat. Normally suitable for pruning. Adaptable to a range of moist, well-drained soil and prefers full or part shade. Dandarrga Nursery Native Species Labels A - M Basket Grass (Lomandra longifolia labill) Asparagaceae Native grass up to 1.2 m high & over 1m wide Flowers: cream to yellow from late winter to summer. Grows in a range of habitats FIRE RETARDANT SPECIES. -

Approved Conservation Advice for the Monsoon Vine Thickets on the Coastal Sand Dunes of Dampier Peninsula
Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) Approved Conservation Advice for the Monsoon vine thickets on the coastal sand dunes of Dampier Peninsula 1. The Threatened Species Scientific Committee (the Committee) was established under the EPBC Act and has obligations to present advice to the Minister for Sustainability, Environment, Water, Population and Communities (the Minister) in relation to the listing and conservation of threatened ecological communities, including under sections 189, 194N and 266B of the EPBC Act. 2. The Committee provided its advice on the Monsoon vine thickets on the coastal sand dunes of Dampier Peninsula ecological community to the Minister as a draft of this approved conservation advice. In 2013, the Minister accepted the Committee’s advice, adopting it as the approved conservation advice. 3. The Minister amended the list of threatened ecological communities under section 184 of the EPBC Act to include the Monsoon vine thickets on the coastal sand dunes of Dampier Peninsula ecological community in the endangered category. It is noted that the ecological community is also listed as the Monsoon vine thickets on the coastal sand dunes of Dampier Peninsula on the Western Australian list of threatened ecological communities endorsed by the Western Australia Minister for the Environment. 4. The nomination and a draft description for this ecological community were made available for expert and public comment for a minimum of 30 business days. The Committee and Minister had regard to all public and expert comment that was relevant to the consideration of the ecological community. 5. This approved conservation advice has been developed based on the best available information at the time it was approved; this includes scientific literature, advice from consultations, existing plans, records or management prescriptions for this ecological community. -

The 1770 Landscape of Botany Bay, the Plants Collected by Banks and Solander and Rehabilitation of Natural Vegetation at Kurnell
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Hochschulschriftenserver - Universität Frankfurt am Main Backdrop to encounter: the 1770 landscape of Botany Bay, the plants collected by Banks and Solander and rehabilitation of natural vegetation at Kurnell Doug Benson1 and Georgina Eldershaw2 1Botanic Gardens Trust, Mrs Macquaries Rd Sydney 2000 AUSTRALIA email [email protected] 2Parks & Wildlife Division, Dept of Environment and Conservation (NSW), PO Box 375 Kurnell NSW 2231 AUSTRALIA email [email protected] Abstract: The first scientific observations on the flora of eastern Australia were made at Botany Bay in April–May 1770. We discuss the landscapes of Botany Bay and particularly of the historic landing place at Kurnell (lat 34˚ 00’ S, long 151˚ 13’ E) (about 16 km south of central Sydney), as described in the journals of Lieutenant James Cook and Joseph Banks on the Endeavour voyage in 1770. We list 132 plant species that were collected at Botany Bay by Banks and Daniel Solander, the first scientific collections of Australian flora. The list is based on a critical assessment of unpublished lists compiled by authors who had access to the collection of the British Museum (now Natural History Museum), together with species from material at National Herbarium of New South Wales that has not been previously available. The list includes Bidens pilosa which has been previously regarded as an introduced species. In 1770 the Europeans set foot on Aboriginal land of the Dharawal people. Since that time the landscape has been altered in response to a succession of different land-uses; farming and grazing, commemorative tree planting, parkland planting, and pleasure ground and tourist visitation. -

Cunninghamia Date of Publication: April 2020 a Journal of Plant Ecology for Eastern Australia
Cunninghamia Date of Publication: April 2020 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) A Systematic Flora Survey, Floristic Classification and High-Resolution Vegetation Map of Lord Howe Island Paul Sheringham 1*, Peter Richards2, Phil Gilmour3, Jill Smith1 and Ernst Kemmerer 4 1 Department of Planning, Industry and Environment, Locked Bag 914 COFFS HARBOUR NSW 2450 2 17 Coronation Avenue, SAWTELL NSW 2452 3 523 Roses Rd, GLENIFFER, NSW 2454 4 Cradle Coast NRM, PO Box 338, BURNIE TAS 7320 * Author for correspondence: [email protected] Abstract: The present study took advantage of the availability of high resolution ADS40 digital imagery to 1) systematically resample the vegetation of the Lord Howe Island Group (LHIG, excluding Ball’s Pyramid); 2) conduct a numerical analysis of the floristic data; 3) map vegetation extent and the distribution of vegetation communities and 4) compare the resultant classification and mapping with those of Pickard (1983). In July 2013, a total of 86 full floristic and 105 rapid floristic sites were sampled across the island, based on a stratified random sampling design. A hierarchical agglomerative clustering strategy (Flexible UPGMA) and Bray-Curtis dissimilarity coefficient with default beta, along with nearest neighbour analysis to identify anomalous site allocations, was used to analyze the floristic data. In total 33 vegetation communities were delineated and mapped: 19 mapping units from the full floristic analysis; 7 variants identified within five of the above 19 groups; 3 mapping units from analysis of canopy- only floristic data; and 4 mapping units recognised in previous studies that are mapped but were not sampled in this survey.