Expanded Concept and Revised Taxonomy of the Milliped Family
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity of Millipedes Along the Northern Western Ghats
Journal of Entomology and Zoology Studies 2014; 2 (4): 254-257 ISSN 2320-7078 Diversity of millipedes along the Northern JEZS 2014; 2 (4): 254-257 © 2014 JEZS Western Ghats, Rajgurunagar (MS), India Received: 14-07-2014 Accepted: 28-07-2014 (Arthropod: Diplopod) C. R. Choudhari C. R. Choudhari, Y.K. Dumbare and S.V. Theurkar Department of Zoology, Hutatma Rajguru Mahavidyalaya, ABSTRACT Rajgurunagar, University of Pune, The different vegetation type was used to identify the oligarchy among millipede species and establish India P.O. Box 410505 that millipedes in different vegetation types are dominated by limited set of species. In the present Y.K. Dumbare research elucidates the diversity of millipede rich in part of Northern Western Ghats of Rajgurunagar Department of Zoology, Hutatma (MS), India. A total four millipedes, Harpaphe haydeniana, Narceus americanus, Oxidus gracilis, Rajguru Mahavidyalaya, Trigoniulus corallines taxa belonging to order Polydesmida and Spirobolida; 4 families belongs to Rajgurunagar, University of Pune, Xystodesmidae, Spirobolidae, Paradoxosomatidae and Trigoniulidae and also of 4 genera were India P.O. Box 410505 recorded from the tropical or agricultural landscape of Northern Western Ghats. There was Harpaphe haydeniana correlated to the each species of millipede which were found in Northern Western Ghats S.V. Theurkar region of Rajgurunagar. At the time of diversity study, Trigoniulus corallines were observed more than Senior Research Fellowship, other millipede species, which supports the environmental determinism condition. Narceus americanus Department of Zoology, Hutatma was single time occurred in the agricultural vegetation landscape due to the geographical location and Rajguru Mahavidyalaya, habitat differences. Rajgurunagar, University of Pune, India Keywords: Diplopod, Northern Western Ghats, millipede diversity, Narceus americanus, Trigoniulus corallines 1. -

Arthropod Diversity and Conservation in Old-Growth Northwest Forests'
AMER. ZOOL., 33:578-587 (1993) Arthropod Diversity and Conservation in Old-Growth mon et al., 1990; Hz Northwest Forests complex litter layer 1973; Lattin, 1990; JOHN D. LATTIN and other features Systematic Entomology Laboratory, Department of Entomology, Oregon State University, tural diversity of th Corvallis, Oregon 97331-2907 is reflected by the 14 found there (Lawtt SYNOPSIS. Old-growth forests of the Pacific Northwest extend along the 1990; Parsons et a. e coastal region from southern Alaska to northern California and are com- While these old posed largely of conifer rather than hardwood tree species. Many of these ity over time and trees achieve great age (500-1,000 yr). Natural succession that follows product of sever: forest stand destruction normally takes over 100 years to reach the young through successioi mature forest stage. This succession may continue on into old-growth for (Lattin, 1990). Fire centuries. The changing structural complexity of the forest over time, and diseases, are combined with the many different plant species that characterize succes- bances. The prolot sion, results in an array of arthropod habitats. It is estimated that 6,000 a continually char arthropod species may be found in such forests—over 3,400 different ments and habitat species are known from a single 6,400 ha site in Oregon. Our knowledge (Southwood, 1977 of these species is still rudimentary and much additional work is needed Lawton, 1983). throughout this vast region. Many of these species play critical roles in arthropods have lx the dynamics of forest ecosystems. They are important in nutrient cycling, old-growth site, tt as herbivores, as natural predators and parasites of other arthropod spe- mental Forest (HJ cies. -

FR-1995-02-28.Pdf
2±28±95 Tuesday Vol. 60 No. 39 February 28, 1995 Pages 10789±11016 Briefings on How To Use the Federal Register For information on briefings in Washington, DC, and Dallas, TX, see announcement on the inside cover of this issue. federal register 1 II Federal Register / Vol. 60, No. 39 / Tuesday, February 28, 1995 SUBSCRIPTIONS AND COPIES PUBLIC Subscriptions: Paper or fiche 202±512±1800 FEDERAL REGISTER Published daily, Monday through Friday, Assistance with public subscriptions 512±1806 (not published on Saturdays, Sundays, or on official holidays), by Online: the Office of the Federal Register, National Archives and Records Telnet swais.access.gpo.gov, login as newuser <enter>, no Administration, Washington, DC 20408, under the Federal Register > Act (49 Stat. 500, as amended; 44 U.S.C. Ch. 15) and the password <enter ; or use a modem to call (202) 512±1661, login as swais, no password <enter>, at the second login as regulations of the Administrative Committee of the Federal Register > > (1 CFR Ch. I). Distribution is made only by the Superintendent of newuser <enter , no password <enter . Documents, U.S. Government Printing Office, Washington, DC Assistance with online subscriptions 202±512±1530 20402. Single copies/back copies: The Federal Register provides a uniform system for making Paper or fiche 512±1800 available to the public regulations and legal notices issued by Assistance with public single copies 512±1803 Federal agencies. These include Presidential proclamations and Executive Orders and Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress and other Federal agency documents of public interest. -

A New Genus of the Millipede Tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean Region
European Journal of Taxonomy 70: 1-12 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2013.70 www.europeanjournaloftaxonomy.eu 2013 · Lazányi E. & Vagalinski B. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:5E23F454-2A68-42F6-86FB-7D9952B2FE7B A new genus of the millipede tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean region Eszter LAZÁNYI1,4 & Boyan VAGALINSKI2,3,5 1 Corresponding author: Department of Zoology, Hungarian Natural History Museum, Baross u. 13, H-1088 Budapest, Hungary. E-mail: [email protected] 2 Faculty of Biology, Sofia University, 8 Dragan Tsankov Blvd., 1164 Sofia, Bulgaria. E-mail: [email protected] 3 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Yurii Gagarin Street, 1113, Sofia, Bulgaria. 4 urn:lsid:zoobank.org:author:02DB48F1-624C-4435-AF85-FA87168CD85A 5 urn:lsid:zoobank.org:author:973B8725-039E-4F29-8D73-96A7F52CF934 Abstract. A new genus of the julid tribe Brachyiulini, Enghophyllum gen. nov., is described, comprising two species from Greece. The type-species, E. naxium (Verhoeff, 1901) comb. nov. (ex Megaphyllum Verhoeff, 1894), appears to be rather widespread in the Aegean: it is known from Antiparos Island and Naxos Island (the type locality), both in the Cyclades, as well as East Mavri Islet, Dodecanese Archipelago (new record). The vulva of E. naxium is described for the first time. In addition,E. sifnium gen. et sp. nov. is described based on a single adult male from Sifnos Island, Cyclades. The new genus is distinct from other genera of the Brachyiulini mainly by its peculiar gonopod structure, apparently disjunct and at least mostly apomorphous: (1) promeres broad, shield-like, in situ protruding mostly posteriad, completely covering the opisthomeres and gonopodal sinus; (2) transverse muscles and coxal apodemes of promere fully reduced; (3) opisthomere with three differentiated processes, i.e., lateral, basal posterior and apical posterior; (4) solenomere rather simple, tubular. -

Centre International De Myriapodologie
N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Mus6umNationald'HistoireNaturelle,Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-75231 ParisCedex05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISIES REPE&TORIEES PANS LA BASE PASCAL DE L' INIST 1995 N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Museum National d'Histoire N aturelle, Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-7 5231 Paris Cedex 05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISTES REPERTORIEES DANS LA BASE PASCAL DE L' INIST 1995 SOMMAIRE CONTENTS ZUSAMMENFASSUNG Pages Seite lOth INTERNATIONAL CONGRESS OF MYRIAPODOLOGY .................................. 1 9th CONGRES INTERNATIONAL DE MYRIAPODOLOGIE.................................................... 1 Contacter le Secretariat permanent par E-M AIL & FA X............................................................ 1 The Proceedings of the 9th International Congress of Myriapodology...................... 2 MILLEPATTIA, sommaire .du prochain bulletin....................................................................... 2 Obituary: Colin Peter FAIRHURST (1942-1994) ............................................................. 3 BULLETIN of the -

REPORT to the CHURCH
REPORT to the CHURCH All Saints’ Day 2011 Domestic and Foreign Missionary Society TABLE of CONTENTS Introduction 4 Office of the Presiding Bishop 5 President of the House of Deputies 6 Chief Operating Officer 7 Mission Department 8 Diversity, Social, and Environmental Ministries Team 9 Office of Asiamerican Ministries 10 Office of Black Ministries 11 Office of Economic and Environmental Affairs 12 Office of Social and Economic Justice 13 Office of Latino/Hispanic Ministries 14 Office of Native American and Indigenous Ministries 15 Diocesan and Congregational Ministries Team 16 Office of Transition Ministry 17 Church Planting, Ministry Redevelopment, 18 and Fresh Expressions of Ministry Office of Congregational Vitality 19 Office of Stewardship 20 Office of Congregational Research 21 Office of Global Partnerships 22 Partnership Office for Africa 23 Partnership Office for Asia and the Pacific 24 Partnership Office for Latin America and the Caribbean 25 Partnership Office for the Middle East 26 Partnership Office for Province IX 27 United Thank Offering 28 Office of Mission Personnel 29 Office of Global Relations 30 Office of Global Networking 31 Office of Ecumenical and Interreligious Relationships 32 Formation and Vocation Team 33 Office of Youth Ministries 34 Office of Lifelong Christian Formation 35 Office of Campus Ministries 36 Office of Young Adult Ministries 37 Office of Government Relations 38 Episcopal Migration Ministries 39 Grants and Scholarships 40 Office of Mission Funding 41 2 Office of Pastoral Development 42 Office of Armed Services and Federal Ministries 43 Archives of the Episcopal Church 44 Office of Communication 45 Information Technology 46 Office of Finance 47 Human Resources 48 Building Services 49 Office of the General Convention 50 3 We, the staff of the Domestic and Foreign Missionary Society, working from New York City and 15 other locations in the United States and abroad, are pleased to offer this report to the church we are privileged to serve. -

Apheloria Polychroma, a New Species of Millipede from the Cumberland Mountains (Polydesmida: Xystodesmidae) PAUL E. MAREK1*
Apheloria polychroma, a new species of millipede from the Cumberland Mountains (Polydesmida: Xystodesmidae) PAUL E. MAREK1*, JACKSON C. MEANS1, DEREK A. HENNEN1 1Virginia Polytechnic Institute and State University, Department of Entomology, Blacksburg, Virginia 24061, U.S.A. *Corresponding author, email: [email protected] Abstract Millipedes of the genus Apheloria occur in temperate broadleaf forests throughout eastern North America and west of the Mississippi River in the Ozark and Ouachita Mountains. Chemically defended with toxins made up of cyanide and benzaldehyde, the genus is part of a community of xystodesmid millipedes that compose several Müllerian mimicry rings in the Appalachian Mountains. We describe a model species of these mimicry rings, Apheloria polychroma n. sp., one of the most variable in coloration of all species of Diplopoda with more than six color morphs, each associated with a separate mimicry ring. Keywords: aposematic, Appalachian, Myriapoda, taxonomy, systematics Introduction Millipedes in the family Xystodesmidae are most diverse in the Appalachian Mountains where about half of the family’s species occur. In the New World, the family is distributed throughout eastern and western North America and south to El Salvador (Marek et al. 2014, Marek et al. 2017). Xystodesmidae occur in the Old World in the Mediterranean, the Russian Far East, Japan, western and eastern China, Taiwan and Vietnam. Taxa include species that are bioluminescent (genus Motyxia) and highly gregarious (genera Parafontaria and Pleuroloma); some form Müllerian mimicry rings. Despite their fascinating biology and critical ecological function as native decomposers in broadleaf deciduous forests in the U.S., their alpha-taxonomy is antiquated, and scores of new species remain undescribed. -
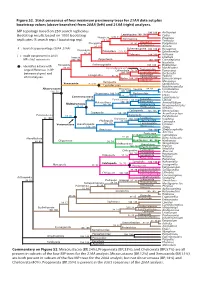
Figure S2. Strict Consensus of Four Maximum Parsimony Trees for 21AA Data Set Plus Bootstrap Values (Above Branches) from 20AA (Left) and 21AA (Right) Analyses
Figure S2. Strict consensus of four maximum parsimony trees for 21AA data set plus bootstrap values (above branches) from 20AA (left) and 21AA (right) analyses. MP topology based on 250 search replicates. 100 100 Antheraea Bootstrap results based on 1000 bootstrap Lepidoptera 100 100 Cydia Neoptera [23] 44 Dermaptera Prodoxus replicates (5 search reps / bootstrap rep). 68 66 Forficula Blattodea Pterygota 92 96 Periplaneta Orthoptera 96 97 Acheta # : bootstrap percentage (20AA 21AA) Ephemeroptera Dicondylia 100 100 Hexagenia Paleoptera [37] 52 Ephemerella 98 98 Odonata 100 100 Ischnura [ ] : node not present in 20AA Insecta Libellula MP strict consensus 100 100 Zygentoma 100 100 Ctenolepisma Nicoletia Archaeognatha : Identifies 6 taxa with Hexapoda 100 100 Pedetontus 89 86 Machiloides Entomobryomorpha Tomoceridae large differences in BP Collembola 70 76 Tomocerus between degen1 and 100 100 Entomobryidae Orchesella Entognatha 82 82 Poduridae Podura 20AA analyses. Diplura 100 100 Campodeidae Japygidae Eumesocampa Remipedia Metajapyx Xenocarida [31] 51 Speleonectes Cephalocarida Hutchinsoniella Altocrustacea Thoracica Sessilia 88 83 Semibalanus [14] 31 100 100 Chthamalus Thecostraca 100 100 Pedunculata Lepas Communostraca Rhizocephala Loxothylacus 96 89 Eumalacostraca 84 85 Eucarida Libinia Malacostraca 100 100 Peracarida Armadillidium Multicrustacea 100 100 Hoplocarida 69 84 Neogonodactylus Phyllocarida Nebalia Cyclopoida 100 100 Mesocyclops Copepoda 100 100 Acanthocyclops Pancrustacea 65 82 Calanoida Eurytemora 96 100 Diplostraca 100 100 -

Download Download
INSECTA MUNDI A Journal of World Insect Systematics 0238 Snoqualmia, a new polydesmid milliped genus from the northwestern United States, with a description of two new species (Diplopoda, Polydesmida, Polydesmidae) William A. Shear Department of Biology Hampden-Sydney College Hampden-Sydney, VA 23943-0096 U.S.A. Date of Issue: June 15, 2012 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL William A. Shear Snoqualmia, a new polydesmid milliped genus from the northwestern United States, with a description of two new species (Diplopoda, Polydesmida, Polydesmidae) Insecta Mundi 0238: 1-13 Published in 2012 by Center for Systematic Entomology, Inc. P. O. Box 141874 Gainesville, FL 32614-1874 USA http://www.centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non-marine arthropod. Topics considered for publication include systematics, taxonomy, nomencla- ture, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book re- views or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources including the Zoological Record, CAB Abstracts, etc. Insecta Mundi is published irregularly throughout the year, with completed manu- scripts assigned an individual number. Manuscripts must be peer reviewed prior to submission, after which they are reviewed by the editorial board to ensure quality. One author of each submitted manu- script must be a current member of the Center for Systematic Entomology. -

Asian Spirituality of Christian Stewardship (Keynote Address of the Rev
Asian Spirituality of Christian Stewardship (Keynote Address of the Rev. Dr. Winfred B. Vergara, Missioner for Asiamerica Ministry of the Episcopal Church Center at the diocesan EAM Consultation of the Diocese of California, held in Christ Episcopal Church, Alameda, California on June 10-11, 2011) Introduction This gathering today, here in this Diocese of California has a triple significance for me: first, it is here, in this diocese where the first national Episcopal Asiamerica Ministry Consultation was held in 1974; second, it is here, in this Diocese, where I was installed as the second missioner for Asiamerica Ministry in 2004; and third, it is here now, in this Diocese that this first diocesan-wide EAM Consultation is ever held. The end of all exploring,” wrote the English poet, T. S. Eliot, “will be to arrive where you started and know the place for the first time.” Or as one Chinese proverb says, “If you just stay in the same place for as long as you can, you will finally see the world coming back to you.” So I feel like, today, it is “dejavu” – all coming back again, as if, for the first time. You‟ve assigned me to speak on “Asian Spirituality of Christian Stewardship.” Let me arranged this theme on three chapters: first, what is spirituality; second, what is Asian; and third, what is Christian stewardship. Then, I will sum up on how we can arrive at a contextual teaching on Christian Stewardship. First, what is Spirituality? A story is told of a parrot which was fond of speaking bad words. -

B a N I S T E R I A
B A N I S T E R I A A JOURNAL DEVOTED TO THE NATURAL HISTORY OF VIRGINIA ISSN 1066-0712 Published by the Virginia Natural History Society The Virginia Natural History Society (VNHS) is a nonprofit organization dedicated to the dissemination of scientific information on all aspects of natural history in the Commonwealth of Virginia, including botany, zoology, ecology, archaeology, anthropology, paleontology, geology, geography, and climatology. The society’s periodical Banisteria is a peer-reviewed, open access, online-only journal. Submitted manuscripts are published individually immediately after acceptance. A single volume is compiled at the end of each year and published online. The Editor will consider manuscripts on any aspect of natural history in Virginia or neighboring states if the information concerns a species native to Virginia or if the topic is directly related to regional natural history (as defined above). Biographies and historical accounts of relevance to natural history in Virginia also are suitable for publication in Banisteria. Membership dues and inquiries about back issues should be directed to the Co-Treasurers, and correspondence regarding Banisteria to the Editor. For additional information regarding the VNHS, including other membership categories, annual meetings, field events, pdf copies of papers from past issues of Banisteria, and instructions for prospective authors visit http://virginianaturalhistorysociety.com/ Editorial Staff: Banisteria Editor Todd Fredericksen, Ferrum College 215 Ferrum Mountain Road Ferrum, Virginia 24088 Associate Editors Philip Coulling, Nature Camp Incorporated Clyde Kessler, Virginia Tech Nancy Moncrief, Virginia Museum of Natural History Karen Powers, Radford University Stephen Powers, Roanoke College C. L. Staines, Smithsonian Environmental Research Center Copy Editor Kal Ivanov, Virginia Museum of Natural History Copyright held by the author(s). -

Ordinal-Level Phylogenomics of the Arthropod Class
Ordinal-Level Phylogenomics of the Arthropod Class Diplopoda (Millipedes) Based on an Analysis of 221 Nuclear Protein-Coding Loci Generated Using Next- Generation Sequence Analyses Michael S. Brewer1,2*, Jason E. Bond3 1 Department of Environmental Science, Policy, and Management, University of California Berkeley, Berkeley, California, United States of America, 2 Department of Biology, East Carolina University, Greenville, North Carolina, United States of America, 3 Department of Biological Sciences and Auburn University Museum of Natural History, Auburn University, Auburn, Alabama, United States of America Abstract Background: The ancient and diverse, yet understudied arthropod class Diplopoda, the millipedes, has a muddled taxonomic history. Despite having a cosmopolitan distribution and a number of unique and interesting characteristics, the group has received relatively little attention; interest in millipede systematics is low compared to taxa of comparable diversity. The existing classification of the group comprises 16 orders. Past attempts to reconstruct millipede phylogenies have suffered from a paucity of characters and included too few taxa to confidently resolve relationships and make formal nomenclatural changes. Herein, we reconstruct an ordinal-level phylogeny for the class Diplopoda using the largest character set ever assembled for the group. Methods: Transcriptomic sequences were obtained from exemplar taxa representing much of the diversity of millipede orders using second-generation (i.e., next-generation or high-throughput) sequencing. These data were subject to rigorous orthology selection and phylogenetic dataset optimization and then used to reconstruct phylogenies employing Bayesian inference and maximum likelihood optimality criteria. Ancestral reconstructions of sperm transfer appendage development (gonopods), presence of lateral defense secretion pores (ozopores), and presence of spinnerets were considered.