J&J's $30Bn for Actelion Buys Immediate and Longer-Term Value
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

Johnson & Johnson
JOHNSON & JOHNSON FORM 10-K (Annual Report) Filed 02/22/13 for the Period Ending 12/30/12 Address ONE JOHNSON & JOHNSON PLZ NEW BRUNSWICK, NJ 08933 Telephone 732-524-2455 CIK 0000200406 Symbol JNJ SIC Code 2834 - Pharmaceutical Preparations Industry Biotechnology & Drugs Sector Healthcare Fiscal Year 12/12 http://www.edgar-online.com © Copyright 2013, EDGAR Online, Inc. All Rights Reserved. Distribution and use of this document restricted under EDGAR Online, Inc. Terms of Use. UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 30, 2012 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

SEC Form 10-K Annual Report
e10vk 10-K 1 y80744e10vk.htm FORM 10-K Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended January 3, 2010 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No þ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). -
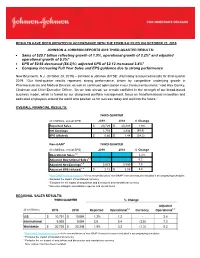
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

Management Liability Focus Johnson & Johnson 1
Insured Profile Report – Management Liability Focus Johnson_________________________________________________________________________ & Johnson Company Profile Credit Details Location 1 Johnson and Johnson Plz Overall Credit Risk High Risk New Brunswick, NJ www.jnj.com Number of Legal Derogatory 84 Company Type Public Items Liability Amount $322,285.00 Formerly Known As N/A Experian Intelliscore 2.57 SIC Code 2834 SIC Code Description Pharmaceutical Preparations Experian Intelliscore Percentile 2.00 % of companies score lower and have higher credit risk Established 1955 Experian Commercial IntelliscoreSM is an all-industry commercial model using business information to predict business risk. Its Sales (in millions) $65,030.00 predictiveness is among the best on the market today The objective of the Commercial Intelliscore Model is to predict seriously Employees 117,900 derogatory payment behavior. Possible score range from 0 to 100, where 0 is high risk and 100 is low risk Total OSHA Violations 18 -Liability Amount is the total dollar amount of debtor’s legal liability, OSHA is an arm of the Department of Labor that conducts inspections of company including accounts in collection, tax liens,judgments and/or bankruptcies facilities with the goal of preventing work-related injuries, illnesses and deaths. -The Number of Legal Derogatory items are the sum of Tax-Lien Worksites that do not meet health and/or safety standards at the time of inspection may Count, Bankruptcy,Judgment, Collection-Counter and UCC Derog receive an OSHA violation. Total FDA NDC Drugs 177 The total number of FDA Drugs filed in the FDA NDC Drug Database. Business Description Johnson & Johnson is engaged in the research and development, manufacture and sale of a range of products in the healthcare field. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Lna 2006 Profiles J.Qxp
1 | Advertising Age | June 26, 2006 SpecialSpecial ReportReport:100 Profiles LEADING NATIONAL ADVERTISERSSupplement SUPPLEMENT June 26, 2006 100 LEADING NATIONAL ADVERTISERS Profiles of the top 100 U.S. marketers in this 51st annual ranking INSIDE TOP 100 RANKING COMPANY PROFILES SPONSORED BY The nation’s leading marketers Lead marketing personnel, ranked by U.S. advertising brands, agencies, agency expenditures for 2005. contacts, as well as advertising Includes data from TNS Media spending by media and brand, Intelligence and Ad Age’s sales, earnings and more for proprietary estimates of the country’s 100 largest unmeasured spending. PAGE 8 advertisers PAGE 10 This document, and information contained therein, is the copyrighted property of Crain Communications Inc. and The Ad Age Group (© Copyright 2006) and is for your personal, non-commercial use only. You may not reproduce, display on a website, distribute, sell or republish this document, or information contained therein, without prior written consent of The Ad Age Group. Are proud to connect you with the leading CMOs See all the interviews at adage.com/point LAUNCHING JUNE 28 © 2006 Crain Communications Inc. www.adage.com 3 | Advertising Age | June 26, 2006 Special Report 100 LEADING NATIONAL ADVERTISERS SUPPLEMENT ABOUT THIS PROFILE EDITION THE 51ST ANNUAL 100 Leading National the Top 100 ($40.13 billion) and for all measured spending in 18 national media, Advertisers Report crowned acquisition- advertisers ($122.79 billion) in the U.S. Yellow Pages Association contributed ladened Procter & Gamble Co. as the top U.S. ad spending by ad category: This spending in Yellow Pages and TNS Marx leader, passing previous kingpen General chart (Page 6) breaks out 18 measured Promotion Intelligence provided free- Motors Corp. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Bestccoorporations
Navoba BUY VETERAN CORPORATE GOVERNMENT FRANCHISE YOUR BIZ COMMUNITY best corporations HUGE CORPORATE for vETERAN-OWNED By Matthew Pavelek CONTRACTING n 1999, the veteran-owned of their way to use VOBs. business movement took In 2007, approximately 10 a giant leap forward with percent of Fortune 1000 companies legislation requiring the sought veteran-owned suppliers. Ifederal government to recognize Through NaVOBA’s efforts, that vetrepreneurs and setting a mandate number has grown by more than OPPORTUNITIES to buy from businesses owned 60 percent. Today, more than 160 by service-disabled veterans. In Fortune 1000 companies maintain 2005, NaVOBA launched Veterans programs designed to use VOBs as Business Journal to bring the VOB preferred vendors in their supplier movement to corporate America. diversity eff orts. While the federal government is Retail colossus Walmart, FOR the world’s single largest purchaser consumer products behemoth of goods and services, the potential Proctor and Gamble, and innovative for vetrepreneurs selling to all of tech giant Apple, Inc. all buy veteran corporate America dwarfs the federal because it’s good business. Johnson opportunity. & Johnson, earning a place among YOUR BUSINESS Unlike the federal government NaVOBA’s Best 10 Corporations for and its prime contractors, many Veteran-Owned Businesses for 2011 states, and some local and municipal and 2012, provides further evidence governments, corporate America is that the VOB movement is advancing NaVOBA is proud to honor the 10 Best Corporationsporations not mandated by law to use veteran- well beyond the beltway. for Veteran-Owned Businesses for 2012. owned suppliers. Just like any Earning the honor as one of business, large corporations exist to NaVOBA’s 10 Best Corporations for turn a profi t. -

Caring for the World . . .One Person at a Time™ Inspires and Unites the People of Johnson & Johnson
OUR CARING TRANSFORMS 2007 Annual Report Caring for the world . .one person at a time™ inspires and unites the people of Johnson & Johnson. We embrace research and science—bringing innovative ideas, products and services to advance the health and well-being of people. Employees of the Johnson & Johnson Family of Companies work with partners in health care to touch the lives of over a billion people every day, throughout the world. The people in our more than 250 companies come to work each day inspired by their personal knowledge that their caring transforms people’s lives . one person at a time. On the following pages, we invite you to see for yourself. Our Caring Transforms ON THE COVER Johnson & Johnson is founding sponsor and continues to support Safe Kids Worldwide®. For 20 years the organization has grown, now teaching prevention as a way to save children’s lives in 17 countries around the world. In Brazil, Nayra Yara da Paz de Jesus carefully washes her hands, a safe, healthy habit she and other children are learning from a local Safe Kids® program. Find out more in our story on page 22. C H A I R M A N ’ S L E T T E R To Our Shareholders Caring for the health and well-being of people throughout the world is an extraordinary business. It is a business where people are passionate about their work, because it matters. It matters to their families, to their communities and to the world. It is a business filled with tremendous opportunity for leadership and growth in the 21st century; a business where unmet needs still abound and where people around the world WILLIAM C. -

The Leading Source of Diabetes Business News It's Complicated
The Leading Source of Diabetes Business News It’s Complicated January/February 2011 • No. 105 The diabetes business used to be fairly straightforward. It’s a lot more complicated now, but that’s not a bad thing. Take insulin. After it was commercialized in the early 1920s, the goal was clear: sell more insulin. But look at today’s three major insulin makers: Eli Lilly, sanofi-aventis, and Novo Nordisk. For all three, to be sure, insulin is still preeminent. The diabetes divisions of Novo Nordisk and Lilly draw most of their revenue from human insulin and rapid-acting analogs, while sanofi-aventis’ blockbuster basal analog Lantus, as well as its mealtime insulins, continue to drive the bottom line. But all three companies have also developed (or are developing) GLP-1 analogs, Lilly has invested in oral drugs for type 2 diabetes (most recently through a major partnership with Boehringer Ingelheim), and the companies are also developing new therapeutic approaches for different stages of disease progression. The evolution of the insulin makers reflects broader industry trends. As our understanding of diabetes has expanded – and we dig deeper into the molecular complexities of the disease – it’s clear that single- focus large companies can no longer prosper. Ours is an era of individualized treatment that mixes and matches different drugs and technologies that try to meet the unique needs of every person with diabetes. Toward that end, companies are forming partnerships to find complementary therapies and development efforts that will create