Exploratory Study: Excessive Iron Supplementation Reduces Zinc Content in Pork Without Affecting Iron and Copper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Critical Audit on Available Beef and Chicken Edible Offals and Their Prices in Retail Chain Stores Around Gaborone, Botswana
Vol. 9(12), pp. 340-347, December 2018 DOI: 10.5897/IJLP2018.0515 Article Number: 0FDE06F59305 ISSN: 2141-2448 Copyright ©2018 International Journal of Livestock Author(s) retain the copyright of this article Production http://www.academicjournals.org/IJLP Full Length Research Paper A critical audit on available beef and chicken edible offals and their prices in retail chain stores around Gaborone, Botswana Molebeledi Horatius Dambe Mareko*, Molefe Gosetsemang, Thabang Molale Botswana University of Agriculture and Natural Resources (BUAN), Gaborone, Botswana. Received 10 August, 2018; Accepted 24 October, 2018 The study aims to determine the available beef and chicken edible offals and their prices in four major retail stores in Gaborone, Botswana. Traditionally, edible beef and chicken offal were available and sold in rural meat and informal markets around Gaborone, but recently upmarket retail stores of Gaborone sell these products. The study was done over a period of twelve months. Amongst the offals noted in the retail stores were ox tail, tongue, spleen, ox heel, kidneys, intestines, rumen, omasum, liver and ox heart for beef and feet, liver, gizzards, intestines, necks and kidneys for chicken. Offals were cheaper than the cheapest standard beef and chicken cuts being the chuck/brisket or stewing beef for beef and breast for chicken. Green beef offals were generally cheaper than red offals. The most expensive beef offal was ox tail at ~P60.00, and the cheapest offal was ox heel at ~P19.95 (USD1.00 ~ BWP11.00). For chicken, the gizzards were the most expensive at ~P49.45, with the necks being the cheapest at ~P26.59. -

Title: Survey of Microbiological Status of Offal Products from Pork
Title: Survey of Microbiological Status of Offal Products from Pork Processing Facilities in the United States – NPB #16-162 Institution: South Dakota State University. Investigators: Alan Erickson (Principal Investigator), South Dakota State University; William Benjy Mikel, WPF Technical Services; Laura Ruesch SDSU; Jane Christopher-Hennings, SDSU; Monte Fuhrman, Pipestone Veterinary Services; Jonathan Ertl, Sioux Nation Ag Center. Date submitted: 10/31/17 Industry Summary: In the United States, approximately five million metric tons of pork variety meats and other byproducts are generated each year with a large amount of this material being rendered to generate low value products like pet food, meat/bone meal, fat, and grease. An alternative use of the US variety meats would be to market and sell them to consumers in countries like China that prefer strong tasting pork products like the variety meats. The desirability of these products in foreign markets makes them higher value products, which could help increase the value of live hogs for US producers. To be able to market and sell these variety meats in global markets, it is important to understand the microbiological status of these products. Therefore, the objective of the current study was to: Determine the microbiological profile of commonly consumed offal products (liver, heart, kidney, brain and intestine) as currently handled in pork production facilities in the United States. This microbiological profile will include tests for: mesophilic aerobic plate counts (APC), Salmonella, Yersinia enterocolitica, and Toxoplasma gondii. To address this objective, samples of heart, kidney, liver, brain and intestine were obtained from 15 pork processing plants in 10 states found across the Midwestern and Southeastern pork-producing region of the US. -
Scottish Menus
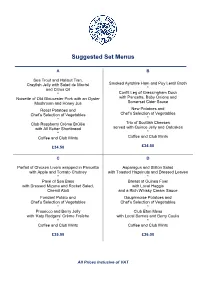
Suggested Set Menus A B Sea Trout and Halibut Tian, Crayfish Jelly with Salad de Maché Smoked Ayrshire Ham and Puy Lentil Broth and Citrus Oil * * Confit Leg of Gressingham Duck Noisette of Old Gloucester Pork with an Oyster with Pancetta, Baby Onions and Somerset Cider Sauce Mushroom and Honey Jus Roast Potatoes and New Potatoes and Chef’s Selection of Vegetables Chef’s Selection of Vegetables * * Club Raspberry Crème Brûlée Trio of Scottish Cheeses with All Butter Shortbread served with Quince Jelly and Oatcakes * * Coffee and Club Mints Coffee and Club Mints £34.50 £34.50 C D Parfait of Chicken Livers wrapped in Pancetta Asparagus and Stilton Salad with Apple and Tomato Chutney with Toasted Hazelnuts and Dressed Leaves * * Pavé of Sea Bass Breast of Guinea Fowl with Dressed Mizuna and Rocket Salad, with Local Haggis Chervil Aïoli and a Rich Whisky Cream Sauce Fondant Potato and Dauphinoise Potatoes and Chef’s Selection of Vegetables Chef’s Selection of Vegetables * * Prosecco and Berry Jelly Club Eton Mess with ‘Katy Rodgers’ Crème Fraîche with Local Berries and Berry Coulis * * Coffee and Club Mints Coffee and Club Mints £35.00 £36.00 All Prices Inclusive of VAT Suggested Set Menus E F Confit of Duck, Guinea Fowl and Apricot Rosettes of Loch Fyne Salmon, Terrine, Pea Shoot and Frissée Salad Lilliput Capers, Lemon and Olive Dressing * * Escalope of Seared Veal, Portobello Mushroom Tournedos of Border Beef Fillet, and Sherry Cream with Garden Herbs Fricasée of Woodland Mushrooms and Arran Mustard Château Potatoes and Chef’s Selection -

The Striped Pig at Home T H I S C H R I S T M a S 'Dining out at Home'
The Striped Pig At Home T H I S C H R I S T M A S 'Dining Out At Home' Our menu has been created with the five key feasting days of the Christmas holiday in mind - Christmas Eve, Christmas Day, Boxing Day, New Year's Eve & New Year's Day. Attached to each menu item, is our day service suggestion based upon over 30 years experience in the hospitality industry. We feel this results in a fantastically well balanced menu to be enjoyed throughout the festive period showcasing the finest local produce. Use them as designed or simply create your own special meal, the choice is yours! And, don't forget - you have the chance to trial your choices in plenty of time before the big day as our first delivery date for this menu is Friday December 11th - tasting is believing! So, sit back and enjoy our culinary journey, whether you are hosting a romantic yuletide lunch or dinner, entertaining all of the family, or sending a meal to your loved ones. We hope that our selections both inspire and make your 'dining out at home' event exceptionally easy and deliciously tasty this Christmas! Delivery Schedule Friday 11th December (all orders must be received by 5:00pm on Tuesday 8th December) Tuesday 22nd December (all orders must be received by 5:00pm on Tuesday 15th December) To Order Call John on 07761 672625 or email [email protected] *please note - a minimum spend of £30 applies* Starters Please note: all starters are portioned & priced for two & cannot be produced individually. -

Breeds of Swine
Breeds of Swine *Eight major breeds of swine produced in the US. *Dark breeds or terminal breeds are used for their production abilities such as meatiness, leanness, durability, growth rate, and feed efficiency. *White breeds or maternal breeds are used for their reproductive abilities such as mothering ability, litter size, and milking ability. Breeds of Swine Dark/Terminal Breeds White/Maternal Breeds Berkshire Chester White Duroc Landrace Hampshire Yorkshire Poland China Spot Berkshire Duroc Hampshire Poland China Spot Chester White Landrace Yorkshire Sex Classes of Swine *Gilt – Any female pig that has not yet given birth. *Sow – A female pig that has given birth. *Boar – An intact male hog kept only for breeding purposes. *Barrow – A castrated male hog used for meat. Scientific Classification of Swine Phylum: Chordata Subphylum: Vertebrata Class: Mammalia Order: Artiodactyla Suborder: Suina Family: Suidae Genus: Sus Species: domesticus Top Ten Swine Producing States 1. Iowa 6. Nebraska 2. North Carolina 7. Missouri 3. Minnesota 8. Oklahoma 4. Illinois 9. Kansas 5. Indiana 10. Ohio Top Five Swine Producing Countries 1. China 2. European Union 3. United States 4. Brazil 5. Canada Pig Vital Signs Normal Body Temperature 101-103°F Normal Heart Rate 60-80 beats/minute Normal Respiration Rate 30-40 breaths/minute Important Breeding Numbers Litter Size: 7-15 pigs Birth Weight: 2-3.5 lbs Weaned at: 21 days Sexual Maturity: 6-8 months # Ideal Number of Teats: 7 per side Estrous Cycle: 21 days (range of 19-21) # Duration of Estrus (heat): 2-3 days Gestation: 114 days (3 months, 3 weeks, 3 days) (range of 112-115) Important Weights of Hogs Birth Weight: 2-3.5 lbs Wean Weight: 15 lbs at 21 days Slaughter Weight: 250 lbs Mature Weight: Male 500-800 lbs Female 400-700 lbs Ear Notching System Right Ear Left Ear Litter Number Individual Pig Number *No more than 2 notches per area except for 81, only one notch. -

Aby's Outdoor Cookbook
Aby’s Outdoor Cookbook Introduction This cookbook is not a part of the Boy Scouts of America program nor do the Boy Scouts of America sanction this book. No representation of such sanctioning is requested, made or implied. The book is the result of more than fifty years experience living outdoors, cooking to please the cook and enjoying the experience. The author made no attempt to calculate calories, nutritional values or cholesterol. He is past seventy years of age and weighs fifteen pounds more than he did at high school graduation. His career path has been varied and perhaps checkered. Whether he was roustabouting in oilfields or managing a major computing center, he has maintained an active and mobile life style, believing hard work (physically hard work) is its own reward. He has failed to diligently follow his belief that “moderation in all things” is the real secret to success, health and abundant living. This tome is intended to provide Scout leaders with background material for cooking outdoors and engaging youth in the joys of this activity. If you are a really good outdoor cook, you will stand out in a crowd. And don’t let anyone tell you it is just the smell of garlic on your hands! At seventy-plus, the author can still identify one hundred fifty birds by sight, forty by flight pattern and fifty by song. He knows more than sixty species of trees by leaf, bark and fruit. He can paddle a canoe, row a boat, carry a pack, sleep on the ground and survive. -

Formulation of Value Added Chicken Meatball with Different Level of Wheat Flour
SAARC J. Agri., 16(1): 205-213 (2018) DOI: http://dx.doi.org/10.3329/sja.v16i1.37435 FORMULATION OF VALUE ADDED CHICKEN MEATBALL WITH DIFFERENT LEVEL OF WHEAT FLOUR M.A. Islam1, M.A. Haque2*, M.J. Ferdwsi3, M.Y. Ali4 and M.A. Hashem1 1Department of Animal Science, Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh 2Department of Biotechnology, Yeungnam University, Gyeongsan 38541, Republic of Korea 3Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh 4Goat and Sheep Production Research Division, Bangladesh Livestock Research Institute, Savar, Bangladesh ABSTRACT The present study was undertaken to evaluate the effect of different levels of wheat flour on the quality characteristics of chicken meatball. Wheat flour which acts as a binding agent of meatball except for control group T1. The meatballs were formulated having 0%, 5%, 10% and 15% wheat flour. The sensory (colour, flavour, texture, juiciness, tenderness, overall acceptability), physicochemical (proximate analysis, pH, cooking loss), biochemical (TBARs, POV, FFA) were analyzed. Treatments were analyzed in a 4×3 factorial experiment in CRD replicated three times per cell. Wheat flour inclusion in meatballs increased cooking yield by reducing weight loss from 27.06 to 26.49%. Among four treatments most preferable colour, odour, tenderness, juiciness was observed significantly (p<0.05) at 15% wheat flour group and the less preferable colour was observed from the control group. The preferablecolourwas observed at 0 days and less preferable colour at 30 day. Meatballs made with the addition of 15% wheat flour had the highest tenderness, overall acceptability, raw pH, cooked pH and lower DM, ash, PV and TBA & showed significant value (p<0.05) The cooked pH was decreased with the increased storage period. -

Investigating the Safety of Meat Co-Products: Microbiology Aspect
Investigating the safety of meat co-products: microbiology aspect A thesis submitted in partial fulfilment of the requirements for the Degree of Master of Science at University of Otago By Linakshi Weerakoon 2020 Abstract Meat co-products (offal) are rich in protein and essential nutrients and have been consumed as delicacies worldwide. China, New Zealand’s largest red meat export market is a country where offal dishes are frequently consumed. As foodborne diseases are a major challenge faced by Chinese consumers, it is important to ensure the quality and safety of offal consumed in China. The objectives of the study were; firstly to investigate the presence of E. coli/ coliforms, Campylobacter jejuni, Salmonella, Clostridium perfringens, Listeria monocytogenes and determine the aerobic plate count (APC) of sheep offal (testes, skirt, liver, tripe, kidney, heart, tail and pizzle) purchased from New Zealand and China using conventional microbiology enumeration methods. Secondly, the distribution of microbial populations present in the sheep offal were investigated using metagenomics. Thirdly, the presence of mycotoxins, aflatoxin B1 (AFB1), deoxynivalenol (DON), zearalenone (ZEA), T-2 toxin and ochratoxin A (OTA) in sheep offal were investigated. Lastly, the decontamination efficiency of chitosan on meat co- products was investigated. Campylobacter jejuni, Salmonella, Clostridium perfringens, Listeria monocytogenes were not present in any of the sheep offal. APC counts obtained for testes, skirt, liver, tripe , kidney, heart, tail and pizzle were 1.85 ± 0.58, 1.65 ± 0.53,1.41 ± 0.28, 1.61± 0.51,1.53 ±0.97, 2.16 ± 0.18 and 2.35 ± 0.46 log CFU/g, respectively for the New Zealand sheep offal and 6.27 ± 0.25, 6.04± 1.53, 6.36 ± 0.72, 5.70 ± 0.92, 7.56 ± 0.58, 7.41 ± 0.56, 7.41 ± 0.45 and 7.44± 1.11 log CFU/g, respectively for the Chinese sheep offal. -

Microbiological Evaluation of Pork Offal Products Collected from Processing Facilities in a Major United States Pork-Producing Region
Brief communication Peer reviewed Microbiological evaluation of pork offal products collected from processing facilities in a major United States pork-producing region Alan K. Erickson, PhD; Monte Fuhrman, DVM; William Benjy Mikel, PhD; Jon Ertl, DVM; Laura L. Ruesch, MS; Debra Murray; Zachary Lau, BS Summary Resumen – Evaluación microbiológica Résumé – Évaluation microbiologique d’abats de porc prélevés dans des établisse- Analysis of 370 offal samples from 15 US de menudencias porcinas recolectadas ments de transformation dans une région pork-processing facilities detected Yersinia de centros procesadores en un región importante de producción porcina de los de production porcine importante aux enterocolitica-positive (2.4%) and Salmonella- États-Unis positive (21.8%) samples and mesophilic Estados Unidos aerobic plate counts > 107 colony-forming El análisis de 370 muestras de menudencias L’analyse de 370 échantillons d’abats prov- units/g (3.2%). A risk assessment showed de 15 centros procesadores de cerdo de EUA enant de 15 établissements de transformation américain a permis de détecter des échan- intestine (20%), brain (21%), liver and heart detectó muestras positivas al Yersinia entero- tillons positifs pour Yersinia enterocolitica (73%), and kidney (87%) sampling batches colitica (2.4%) y positivas a la Salmonella (2.4%) et Salmonella (21.8%) ainsi que des were acceptable for human consumption. (21.8%), y conteo de placa aeróbica de mesó- dénombrements de bactéries mésophiles aéro- filos > 107 unidades/g formadoras de colonias biques > 107 unités formatrices de colonies/g Keywords: swine, offal,Salmonella , Yer- (3.2%). Una evaluación de riesgo mostró que sinia, Toxoplasma (3.2%). Une évaluation du risque a démontré los lotes de muestreo de intestino (20%), cere- que les lots échantillonnés d’intestins (20%), Received: March 20, 2018 bro (21%), hígado y corazón (73%), y riñón de cerveau (21%), de foie et de cœur (73%), Accepted: August 21, 2018 (87%) eran aceptables para consumo humano. -

Eat In. Take Out. Catering
FAMILY + LOVE + BBQ = EAT IN. TAKE OUT. CATERING. WELCOME TO THE PIK N’ PIG! Our family has put 3 generations of love, sweat & tears into the BBQ business, and we are happy to share that commitment (and not to mention the delicious food) with you today. Pik-n-Pig.com facebook.com/piknpig 194 Gilliam-McConnell Rd | Carthage, NC 28327 | Tuesday - Saturday: 11-8 | Sunday: 11-3 910.947.7591 Starters SMOKED WINGS For our newest creation we use fresh local jumbo chicken wings, slow smoked over sweet hickory coals then fried to order. Sauced in your choice of spicy vinegar or honey BBQ and served with our homemade Ranch dressing. $8.50 FRIED PICKLES A local favorite! We bread pickle chips in a seasoned tempura, then lightly fried and served with our homemade Ranch dressing. A must try! $6.50 GARDEN SALAD Get it with... All of our “Q” is hickory smoked Made with fresh, locally grown lettuce, - SMOKED OR GRILLED CHICKEN until melt in the mouth tender... topped with cucumbers, carrots, tomato and red onion. With your choice of - PULLED PORK Being wood fired creates a pink homemade Ranch, Balsamic Vinaigrette, - TURKEY color in the meat that intensifies Italian or Thousand Island Dressing. Add $4.25 $5.25 the flavor and keeps it extra juicy. Sandwiches CLUB SMOKED TURKEY Fresh sliced deli ham, slow smoked QSlow smoked turkey breast seasoned turkey and bacon topped with with our signature rub. Sliced thick, PULLED PORK locally grown lettuce, tomato, mayo piled high on a roll. Served with Seasoned with our signature butt rub. -

Standards for Slaughter of Sheep and Goat and Processing of Sheep And
Standards for slaughter of sheep and goat and processing of sheep and goat meat and sheep and goat offal eligible for export to Japan Export Verification Program This Export Verification Program (EVP) provides the specified products processing requirements and requirements for facilities for the export of sheep and goat meat and sheep and goat offal ∗ to Japan from Spain. This EVP comes in addition to the Spanish and EU regulations but might include some relevant domestic requirements. The Ministry of Agriculture, Fisheries and Food (MAPA) and the Ministry of Health, Consumption and Social Welfare (MSCBS) are the central Spanish competent authorities overseeing the implementation of the EVP in Spain. 1. Purpose This EVP describes the standards that slaughterhouses and processing facilities shall meet in producing sheep and goat meat and sheep and goat offal for export to Japan in order to meet the following objectives: - Ensure removal from ovine and caprine carcasses of all tissues ineligible for export to Japan; - Prevent cross contamination of eligible sheep and goat meat and sheep and goat offal for export to Japan from ineligible tissues during slaughter and/or processing; - Enable verification of compliance with Japan import condition relating to Transmissible Spongiform Encephalopathies (TSEs), in addition to Spanish and EU domestic requirements. 2. Scope This EVP applies to Spanish facilities producing sheep and goat meat and sheep and goat offal* for export to Japan from Spain. The facilities shall meet the specified processing requirements and requirements for facilities for sheep and goat meat and sheep and goat offal for export to Japan from Spain. -

Pork Cut Sheet
Pork Cut Sheet Visit www.edgewoodlocker.com to submit your order online or Call (563) 928-6814 to talk with our experienced staff 609 West Union Street P.O. Box 245 Edgewood, Iowa 52042 (563) 928-6814 www.edgewoodlocker.com Cut Options Loin Pork Chop ½” or ¾” Iowa Chop 1” or 1 ¼” America’s Cut 1” Lard may be chunked, ground or rendered. Any or all cuts may be ground for sausage. Butterfly Chop ½” or ¾” See other side for sausage options. Smoked Chop ½”, ¾” or 1” Country Style Ribs Pork Processing Pork Loin Roast Whole Tenderloin Slaughter Charges Canadian Bacon *Based on carcass weight, per hog* Tenderized Loins Hogs under 170lbs .................................................. $28.00 Marinated Tenderized Loins Hogs 170lbs-199lbs ................................................. $30.00 Picnic Hogs 200lbs-299lbs ................................................. $32.00 Hogs 300lbs and above ........................................ $50.00 Fresh Pork Roast Offal Disposal .......................................................... $10.00 Fresh Pork Steak Off Schedule Surcharge ....................................... $25.00 Fresh Pork Cutlets Smoked Picnic Roast Fresh or Smoked Hock Processing Charges Sliced or Shredded BBQ Pork *Based on carcass weight, per pound* Sliced or Shredded Roast Pork & Gravy Pork Processing ....................................................... $0.67 Butt Half of Hog Surcharge .......................................... $0.04 Fresh Pork Steak Fresh Pork Roast Smoking Charges Fresh Pork Cutlets *Price per pound* Smoked Cottage Bacon Belly, Ham, Hock, Loin, or Shoulder .................... $1.00 Sliced or Shredded BBQ Sliced or Shredded Roast Pork & Gravy Slicing Charges Ham *Price per pound in addition to smoking charge* Smoked Ham Roast Slicing Canadian, Cottage or Picnic Bacon .... $0.25 Smoked Ham Steak Slicing Sandwich or Shaved Ham ....................... $0.25 Smoked Pork Hock Slicing Regular Bacon ........................................... $0.25 Smoked Sliced Sandwich Ham Slicing & Seasoning Flavored Bacon.................