Chapter 3. Liver Anatomy 2018 FINAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor
Human Anatomy (Biology 2) Lecture Notes Updated July 2017 Instructor: Rebecca Bailey 1 Chapter 1 The Human Body: An Orientation • Terms - Anatomy: the study of body structure and relationships among structures - Physiology: the study of body function • Levels of Organization - Chemical level 1. atoms and molecules - Cells 1. the basic unit of all living things - Tissues 1. cells join together to perform a particular function - Organs 1. tissues join together to perform a particular function - Organ system 1. organs join together to perform a particular function - Organismal 1. the whole body • Organ Systems • Anatomical Position • Regional Names - Axial region 1. head 2. neck 3. trunk a. thorax b. abdomen c. pelvis d. perineum - Appendicular region 1. limbs • Directional Terms - Superior (above) vs. Inferior (below) - Anterior (toward the front) vs. Posterior (toward the back)(Dorsal vs. Ventral) - Medial (toward the midline) vs. Lateral (away from the midline) - Intermediate (between a more medial and a more lateral structure) - Proximal (closer to the point of origin) vs. Distal (farther from the point of origin) - Superficial (toward the surface) vs. Deep (away from the surface) • Planes and Sections divide the body or organ - Frontal or coronal 1. divides into anterior/posterior 2 - Sagittal 1. divides into right and left halves 2. includes midsagittal and parasagittal - Transverse or cross-sectional 1. divides into superior/inferior • Body Cavities - Dorsal 1. cranial cavity 2. vertebral cavity - Ventral 1. lined with serous membrane 2. viscera (organs) covered by serous membrane 3. thoracic cavity a. two pleural cavities contain the lungs b. pericardial cavity contains heart c. the cavities are defined by serous membrane d. -

Linear Endoscopic Ultrasound Evaluation of Hepatic Veins
Submit a Manuscript: http://www.f6publishing.com World J Gastrointest Endosc 2018 October 16; 10(10): 283-293 DOI: 10.4253/wjge.v10.i10.283 ISSN 1948-5190 (online) MINIREVIEWS Linear endoscopic ultrasound evaluation of hepatic veins Malay Sharma, Piyush Somani, Chittapuram Srinivasan Rameshbabu Malay Sharma, Piyush Somani, Department of Gastroenterology, Abstract Jaswant Rai Speciality Hospital, Meerut 25001, Uttar Pradesh, India Liver resection surgery can be associated with signi- ficant perioperative mortality and morbidity. Extensive Piyush Somani, Department of Gastroenterology, Thumbay knowledge of the vascular anatomy is essential for Hospital, Dubai 415555, United Arab Emirates successful, uncomplicated liver surgeries. Various imaging techniques like multidetector computed Chittapuram Srinivasan Rameshbabu, Department of Anatomy, tomographic and magnetic resonance angiography are Muzaffarnagar Medical College, Muzaffarnagar 251001, Uttar used to provide information about hepatic vasculature. Pradesh, India Linear endoscopic ultrasound (EUS) can offer a detailed evaluation of hepatic veins, help in assessment of ORCID number: Malay Sharma (0000-0003-2478-9117); Piyush Somani (0000-0002-5473-7265); Chittapuram Srinivasan liver segments and can offer a possible route for EUS Rameshbabu (0000-0002-6505-2296). guided vascular endotherapy involving hepatic veins. A standard technique for visualization of hepatic veins by Author contributions: Sharma M wrote the manuscript; Somani linear EUS has not been described. This review paper P, Rameshbabu CS edited the manuscript; Sharma M, Somani P, describes the normal EUS anatomy of hepatic veins Rameshbabu CS designed the study. and a standard technique for visualization of hepatic veins from four stations. With practice an imaging of Conflict-of-interest statement: Authors declare no conflict of all the hepatic veins is possible from four stations. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Anatomy of the Pig
Hands on Workshop: Lecture for Animal Workshop Anatomy of the pig Jong Man Kim1, Hae Won Lee2 Sungkyunkwan University1, Seoul National University2, Korea Introduction The digestive system of swine has anatomic differences from humans. However, the physiology of digestion remains similar to humans. In spite of the anatomic differences, the pig has been used extensively as a gastro- intestinal model. Most of the classical models involving the digestive system have been related to nutritional stud- ies to study digestion of the pig and for studying human digestive phenomenon. More recently endoscopic and laparoscopic surgical models have been developed and used extensively in the swine. The size and function of structures such as the biliary system and pancreatic duct make them amenable for studying human sized equip- ment and biomaterial implants. Surgical modifications have made the intestinal tract amenable to the study of surgical and chronic fistulation procedures. Laparoscopic surgery has replaced many open operations. The procedures described are commonly performed laparoscopically by many general surgeons but require practice. The porcine model is ideal to train surgeons in laparoscopic procedures since porcine anatomy is generally similar to humans with some minor differences. Liver 1. Morphological feature In the human, the liver morphologically consists of 4 lobes; the left, right, quadrate, and caudate lobes al- though the functional anatomy is more important than the morphological one, which is rarely used in clinical field. Unlikely to the human, the porcine liver consists of 5 lobes; the left lateral and medial, right lateral and medial, and caudate lobes. In the ventral view, 4 lobes are seen; the left lateral, left medial, right medial, and right lateral lobes in sequence from left to right. -

Morphological Basis of Clinical Hepatology;
Gastroenterology & Hepatology: Open Access Review Article Open Access Morphological basis of clinical Hepatology Abstract Volume 10 Issue 4 - 2019 Morphological organization of the liver in humans normally studied at a sufficiently high level. The functions of the liver, which play an important role in the regulation of metabolic Milyukov VE, Sharifova HM, Sharifov ER and adaptive processes was researched in detail, but the dynamics of morphological and Department of Human Anatomy, First Moscow State Medical functional changes in the liver in different diseases isn’t studied enough. However, many University, Russia diseases are accompanied by clinical symptoms that may be due to the lack of functional activity of the liver. Correspondence: Milyukov VE, Department of Human Anatomy, First Moscow State Medical University Moscow, In the modern world, there is a steady growth of both primary liver diseases and secondary Russia, Email [email protected] liver lesions in diseases of other organs and systems. Detailed knowledge of both microanatomy and liver microanatomy, by practitioners and, in particular, by surgeons, Received: June 24, 2019 | Published: August 13, 2019 contribute to the objectification of the choice of treatment tactics and, accordingly, to improve the results of patient treatment with liver diseases. Keywords: liver microanatomy, liver microanatomy, liver diseases Abbreviations: CH, chronic viral hepatitis; AIO, acute Hepatic complications that develop in acute diseases of the intestinal obstruction; SPN, sensory peptidergic -

Hepatocyte and Islet Cell Cotransplantation on Poly-L-Lactide Matrix for the Treatment of Liver Cirrhosis
Hindawi International Journal of Hepatology Volume 2020, Article ID 5410359, 6 pages https://doi.org/10.1155/2020/5410359 Research Article Hepatocyte and Islet Cell Cotransplantation on Poly-L-Lactide Matrix for the Treatment of Liver Cirrhosis Siufui Hendrawan ,1,2 Jennifer Lheman,1 Nuraeni,1 Ursula Weber,1,3 and Hans Ulrich Baer3,4 1Tarumanagara Human Cell Technology Laboratory, Faculty of Medicine, Tarumanagara University, Jakarta 11440, Indonesia 2Department of Biochemistry and Molecular Biology, Faculty of Medicine, Tarumanagara University, Jakarta 11440, Indonesia 3Baermed, Centre of Abdominal Surgery, Hirslanden Clinic, 8032 Zürich, Switzerland 4Department of Visceral and Transplantation Surgery, University of Bern, Switzerland Correspondence should be addressed to Siufui Hendrawan; [email protected] Received 7 April 2020; Revised 26 September 2020; Accepted 7 October 2020; Published 14 October 2020 Academic Editor: Dirk Uhlmann Copyright © 2020 Siufui Hendrawan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The human autologous hepatocyte matrix implant is a promising alternative procedure to counter liver damage. We assessed the outcome of human hepatocytes isolation from cirrhotic liver compared to the clinical and histological scores of disease severity. A total of 11 patients with various clinical scores (CTP and MELD) and histological score (Metavir, fibrosis) of liver cirrhosis were included in the hepatocyte matrix implant clinical phase I study. The liver segment and pancreatic tissue were harvested from each patient, and hepatocytes and cells of islets of Langerhans were isolated. The freshly isolated human hepatocytes were coseeded with the islet cells onto poly(l-lactic acid) (PLLA) scaffolds, cultured, and transplanted back into the patient. -

Human Liver Segments: Role of Cryptic Liver Lobes and Vascular Physiology
www.nature.com/scientificreports OPEN Human liver segments: role of cryptic liver lobes and vascular physiology in the development of Received: 11 October 2017 Accepted: 16 November 2017 liver veins and left-right asymmetry Published: xx xx xxxx Jill P. J. M. Hikspoors1, Mathijs M. J. P. Peeters1, Nutmethee Kruepunga1,2, Hayelom K. Mekonen1, Greet M. C. Mommen1, S. Eleonore Köhler 1,3 & Wouter H. Lamers 1,4 Couinaud based his well-known subdivision of the liver into (surgical) segments on the branching order of portal veins and the location of hepatic veins. However, both segment boundaries and number remain controversial due to an incomplete understanding of the role of liver lobes and vascular physiology on hepatic venous development. Human embryonic livers (5–10 weeks of development) were visualized with Amira 3D-reconstruction and Cinema 4D-remodeling software. Starting at 5 weeks, the portal and umbilical veins sprouted portal-vein branches that, at 6.5 weeks, had been pruned to 3 main branches in the right hemi-liver, whereas all (>10) persisted in the left hemi-liver. The asymmetric branching pattern of the umbilical vein resembled that of a “distributing” vessel, whereas the more symmetric branching of the portal trunk resembled a “delivering” vessel. At 6 weeks, 3–4 main hepatic-vein outlets drained into the inferior caval vein, of which that draining the caudate lobe formed the intrahepatic portion of the caval vein. More peripherally, 5–6 major tributaries drained both dorsolateral regions and the left and right ventromedial regions, implying a “crypto-lobar” distribution. Lobar boundaries, even in non-lobated human livers, and functional vascular requirements account for the predictable topography and branching pattern of the liver veins, respectively. -

Rare Anatomic Variations of the Right Hepatic Biliary System
Surgical and Radiologic Anatomy (2019) 41:1087–1092 https://doi.org/10.1007/s00276-019-02260-5 ANATOMIC VARIATIONS Rare anatomic variations of the right hepatic biliary system Shallu Garg1 · Hemanth Kumar2 · Daisy Sahni1 · T. D. Yadav2 · Anjali Aggarwal1 · Tulika Gupta1 Received: 29 January 2019 / Accepted: 17 May 2019 / Published online: 21 May 2019 © Springer-Verlag France SAS, part of Springer Nature 2019 Abstract Purpose To report rare and clinically signifcant anatomic variations in the biliary drainage of right hepatic lobe. Methods Unique variations in the extra- and intrahepatic biliary drainage of right hepatic lobe were observed in 6 cadaveric livers during dissection on 100 formalin-fxed en bloc cadaveric livers. Results There was presence of aberrant drainage of right segmental and sectorial ducts in four cases and of accessory right posterior sectorial duct in two cases. Conclusions We encountered some extensively complicated biliary drainage of right hepatic lobe, unsuccessful recognition of which can lead to serious biliary complications during hepatobiliary surgeries and biliary interventions. Keywords Hepatic ducts · Biliary anatomy · Liver anatomy · Hepatobiliary surgery Introduction LHD at the hepatic hilum, anterior to the RPV (Fig. 1a). The deviation from this typical biliary anatomy is commonly Precise knowledge of biliary anatomy is a prerequisite for found in clinical practice and has been appropriately classi- obtaining optimal results in ever-increasing complex hepa- fed in the previous studies [3, 15]. However, some new or tobiliary surgeries (e.g., extended hepatic resections, liver extremely rare variations of clinical signifcance still can be transplantation, and laparoscopic cholecystectomy). Biliary encountered and should be documented. We herein report complication remains a major cause of morbidity and mor- six unique cases of complex biliary anatomy that may pose tality, despite improvements in hepatic surgical techniques as one of the important risk factors for bile duct injury dur- [1]. -

Localisation of Focal Liver Lesions to Specific Hepatic Segments — Comparison of Multiphase Spiral CT and MR Imaging
Folia Morphol. Vol. 61, No. 4, pp. 291–297 Copyright © 2002 Via Medica O R I G I N A L ARTICLE ISSN 0015–5659 www.fm.viamedica.pl Localisation of focal liver lesions to specific hepatic segments — comparison of multiphase spiral CT and MR imaging Barbara Bobek-Billewicz1, Edyta Szurowska1, Adam Zapaśnik1, Ewa Iżycka-Świeszewska2, Tomasz Gorycki1, Marek Nowakowski1 1Department of Radiology, Medical University of Gdańsk, Poland 2Department of Pathomorphology, Medical University of Gdańsk, Poland [Received 2 October 2002; Revised 30 October 2002; Accepted 30 October 2002] The purpose of this study was an evaluation of the ability of the mulitiphase spiral CT and MR imaging to localise focal liver lesions referring to specific he- patic segments. The authors studied prospectively 26 focal liver lesions in 26 patients who had undergone spiral CT and MRI before surgery. Multiphase spiral CT included non- contrast scans, hepatic arterial-dominant phase, portal venous — dominant phase and equilibrium phase. MRI was performed in all cases. The following sequenc- es were performed: SE and TSE T1- and T2-weighted images, STIR and dynamic T1-weighted FFE study after i.v. administration of gadolinium (Gd-DTPA). The CT and MR scans were prospectively and independently reviewed by three radiologists for visualisation of hepatic and portal veins and segmental localisa- tion of hepatic lesions. The authors used the right and left main portal veins along with transverse fissu- ra, hepatic veins and gallbladder fossa as landmarks for the tumour localisation to specific hepatic segments. The primary segmental locations of the lesions were correctly determined with CT in 22 of 26 focal liver lesions (85%) and with MR imaging in 24 of 26 lesions (92%). -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
Brain and Central Nervous System
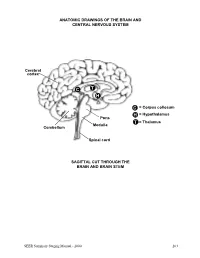
ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM Cerebral cortex C T H C = Corpus collosum H = Hypothalamus Pons T = Thalamus Medulla Cerebellum Spinal cord SAGITTAL CUT THROUGH THE BRAIN AND BRAIN STEM SEER Summary Staging Manual - 2000 263 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM 2 1 3 4 7 5 8 6 SAGITTAL CUT THROUGH THE HUMAN HEAD WITH CEREBRUM IN PLACE The cerebrum is comprised of the: 1 Frontal lobe 2 Parietal lobe 3 Temporal lobe 4 Occipital lobe Other parts of the brain include: 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) 264 SEER Summary Staging Manual - 2000 ANATOMIC DRAWINGS OF THE BRAIN AND CENTRAL NERVOUS SYSTEM A B C D E 7 5 6 8 F SAGITTAL CUT THROUGH THE HUMAN HEAD Internal anatomy of the brain: A Inner surface of right hemisphere of cerebrum B Corpus callosum C Velum interpositum D Middle commissure E Third ventricle F Fourth ventricle Other parts of the brain (as on previous drawing): 5 Pons 6 Medulla (oblongata) 7 Cerebellum 8 Tentorium (cerebelli) SEER Summary Staging Manual - 2000 265 BRAIN AND CEREBRAL MENINGES C70.0, C71.0-C71.9 Supratentorial (S) or Infratentorial (I) C70.0 Cerebral meninges C71.0 Cerebrum ? (S) C71.1 Frontal lobe (S) C71.2 Temporal lobe (S) C71.3 Parietal lobe (S) C71.4 Occipital lobe (S) C71.5 Ventricle, NOS (S) C71.6 Cerebellum, NOS (I) C71.7 Brain stem (I) C71.8 Overlapping lesion of brain ? C71.9 Brain, NOS ? ?See Note 1. SUMMARY STAGE 1 Localized only Supratentorial tumor confined to: Cerebral hemisphere (cerebrum) or meninges of cerebral hemisphere -

A CORROSION CAST STUDY of RAMIFICATION PATTERN of PORTAL VEIN in RIGHT LOBE of HUMAN LIVER Rajput AS *1, Kumari S 2, Mishra GP 3
International Journal of Anatomy and Research, Int J Anat Res 2014, Vol 2(4):791-96. ISSN 2321- 4287 Original Article DOI: 10.16965/ijar.2014.551 A CORROSION CAST STUDY OF RAMIFICATION PATTERN OF PORTAL VEIN IN RIGHT LOBE OF HUMAN LIVER Rajput AS *1, Kumari S 2, Mishra GP 3. *1 Assistant Professor, Department of Anatomy, RIMS, Bareilly, Uttar Pradesh, India. 2 Assistant Professor, Department of Forensic Medicine & Toxicology, Mayo Institute of Medical Sciences, Barabanki, Uttar Pradesh, India. 3 Associate Professor, Department of Anatomy, Career Institute of Medical Sciences and Hospitals, Ghaila, Lucknow, Uttar Pradesh, India. ABSTRACT Objectives: The aim of the study was to know the intrahepatic ramification pattern of portal vein in right lobe of liver & its variations. Methods: 25 human fresh livers were obtained after autopsy and studied by corrosion cast method. Polymeric granules of butyl butyrate were dissolved in acetone and 20% homogenous solution was made. Solution was injected into portal vein and the injected liver was placed in 10 % formal saline for 24 hours at room temperature (20°C) for polymerization of infused butyl butyrate solution. Maceration of liver tissue achieved by whole- organ immersion in 1.8 N KOH solution at 68°C for 24 hrs. Each cast thus obtained was preserved in glycerin and details were studied. Results: The length of the right portal vein varies 0.5 to 1.8 cm (1.2 cm). The right portal vein bifurcated into second order branches - right anterior portal vein (RAPV) & right posterior portal vein (RPPV) in 87 % of the cases, while trifurcated in rest of 13 % of cases.