Opacity and Optical Depth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE EVOLUTION of SOLAR FLUX from 0.1 Nm to 160Μm: QUANTITATIVE ESTIMATES for PLANETARY STUDIES
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by St Andrews Research Repository The Astrophysical Journal, 757:95 (12pp), 2012 September 20 doi:10.1088/0004-637X/757/1/95 C 2012. The American Astronomical Society. All rights reserved. Printed in the U.S.A. THE EVOLUTION OF SOLAR FLUX FROM 0.1 nm TO 160 μm: QUANTITATIVE ESTIMATES FOR PLANETARY STUDIES Mark W. Claire1,2,3, John Sheets2,4, Martin Cohen5, Ignasi Ribas6, Victoria S. Meadows2, and David C. Catling7 1 School of Environmental Sciences, University of East Anglia, Norwich, UK NR4 7TJ; [email protected] 2 Virtual Planetary Laboratory and Department of Astronomy, University of Washington, Box 351580, Seattle, WA 98195, USA 3 Blue Marble Space Institute of Science, P.O. Box 85561, Seattle, WA 98145-1561, USA 4 Department of Physics & Astronomy, University of Wyoming, Box 204C, Physical Sciences, Laramie, WY 82070, USA 5 Radio Astronomy Laboratory, University of California, Berkeley, CA 94720-3411, USA 6 Institut de Ciencies` de l’Espai (CSIC-IEEC), Facultat de Ciencies,` Torre C5 parell, 2a pl, Campus UAB, E-08193 Bellaterra, Spain 7 Virtual Planetary Laboratory and Department of Earth and Space Sciences, University of Washington, Box 351310, Seattle, WA 98195, USA Received 2011 December 14; accepted 2012 August 1; published 2012 September 6 ABSTRACT Understanding changes in the solar flux over geologic time is vital for understanding the evolution of planetary atmospheres because it affects atmospheric escape and chemistry, as well as climate. We describe a numerical parameterization for wavelength-dependent changes to the non-attenuated solar flux appropriate for most times and places in the solar system. -
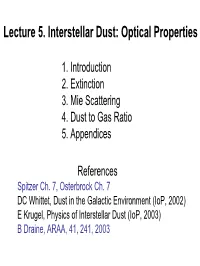
Lecture 5. Interstellar Dust: Optical Properties
Lecture 5. Interstellar Dust: Optical Properties 1. Introduction 2. Extinction 3. Mie Scattering 4. Dust to Gas Ratio 5. Appendices References Spitzer Ch. 7, Osterbrock Ch. 7 DC Whittet, Dust in the Galactic Environment (IoP, 2002) E Krugel, Physics of Interstellar Dust (IoP, 2003) B Draine, ARAA, 41, 241, 2003 1. Introduction: Brief History of Dust Nebular gas long accepted but existence of absorbing interstellar dust controversial. Herschel (1738-1822) found few stars in some directions, later extensively demonstrated by Barnard’s photos of dark clouds. Trumpler (PASP 42 214 1930) conclusively demonstrated interstellar absorption by comparing luminosity distances & angular diameter distances for open clusters: • Angular diameter distances are systematically smaller • Discrepancy grows with distance • Distant clusters are redder • Estimated ~ 2 mag/kpc absorption • Attributed it to Rayleigh scattering by gas Some of the Evidence for Interstellar Dust Extinction (reddening of bright stars, dark clouds) Polarization of starlight Scattering (reflection nebulae) Continuum IR emission Depletion of refractory elements from the gas Dust is also observed in the winds of AGB stars, SNRs, young stellar objects (YSOs), comets, interplanetary Dust particles (IDPs), and in external galaxies. The extinction varies continuously with wavelength and requires macroscopic absorbers (or “dust” particles). Examples of the Effects of Dust Extinction B68 Scattering - Pleiades Extinction: Some Definitions Optical depth, cross section, & efficiency: ext ext ext τ λ = ∫ ndustσ λ ds = σ λ ∫ ndust 2 = πa Qext (λ) Ndust nd is the volumetric dust density The magnitude of the extinction Aλ : ext I(λ) = I0 (λ) exp[−τ λ ] Aλ =−2.5log10 []I(λ)/I0(λ) ext ext = 2.5log10(e)τ λ =1.086τ λ 2. -

ASTR 545 Module 2 HR Diagram 08.1.1 Spectral Classes: (A) Write out the Spectral Classes from Hottest to Coolest Stars. Broadly
ASTR 545 Module 2 HR Diagram 08.1.1 Spectral Classes: (a) Write out the spectral classes from hottest to coolest stars. Broadly speaking, what are the primary spectral features that define each class? (b) What four macroscopic properties in a stellar atmosphere predominantly govern the relative strengths of features? (c) Briefly provide a qualitative description of the physical interdependence of these quantities (hint, don’t forget about free electrons from ionized atoms). 08.1.3 Luminosity Classes: (a) For an A star, write the spectral+luminosity class for supergiant, bright giant, giant, subgiant, and main sequence star. From the HR diagram, obtain approximate luminosities for each of these A stars. (b) Compute the radius and surface gravity, log g, of each luminosity class assuming M = 3M⊙. (c) Qualitative describe how the Balmer hydrogen lines change in strength and shape with luminosity class in these A stars as a function of surface gravity. 10.1.2 Spectral Classes and Luminosity Classes: (a,b,c,d) (a) What is the single most important physical macroscopic parameter that defines the Spectral Class of a star? Write out the common Spectral Classes of stars in order of increasing value of this parameter. For one of your Spectral Classes, include the subclass (0-9). (b) Broadly speaking, what are the primary spectral features that define each Spectral Class (you are encouraged to make a small table). How/Why (physically) do each of these depend (change with) the primary macroscopic physical parameter? (c) For an A type star, write the Spectral + Luminosity Class notation for supergiant, bright giant, giant, subgiant, main sequence star, and White Dwarf. -

Essay on Optics
Essay on Optics by Émilie du Châtelet translated, with notes, by Bryce Gessell published by LICENSE AND CITATION INFORMATION 2019 © Bryce Gessell This work is licensed by the copyright holder under a Creative Commons Attribution-NonCommercial 4.0 International License. Published by Project Vox http://projectvox.org How to cite this text: Du Châtelet, Emilie. Essay on Optics. Translated by Bryce Gessell. Project Vox. Durham, NC: Duke University Libraries, 2019. http://projectvox.org/du-chatelet-1706-1749/texts/essay-on-optics This translation is based on the copy of Du Châtelet’s Essai sur l’Optique located in the Universitätsbibliothek Basel (L I a 755, fo. 230–265). The original essay was transcribed and edited in 2017 by Bryce Gessell, Fritz Nagel, and Andrew Janiak, and published on Project Vox (http://projectvox.org/du-chatelet-1706-1749/texts/essai-sur-loptique). 2 This work is governed by a CC BY-NC 4.0 license. You may share or adapt the work if you give credit, link to the license, and indicate changes. You may not use the work for commercial purposes. See creativecommons.org for details. CONTENTS License and Citation Information 2 Editor’s Introduction to the Essay and the Translation 4 Essay on Optics Introduction 7 Essay on Optics Chapter 1: On Light 8 Essay on Optics Chapter 2: On Transparent Bodies, and on the Causes of Transparence 11 Essay on Optics Chapter 3: On Opacity, and on Opaque Bodies 28 Essay on Optics Chapter 4: On the Formation of Colors 37 Appendix 1: Figures for Essay on Optics 53 Appendix 2: Daniel II Bernoulli’s Note 55 Appendix 3: Figures from Musschenbroek’s Elementa Physicae (1734) 56 Appendix 4: Figures from Newton’s Principia Mathematica (1726) 59 3 This work is governed by a CC BY-NC 4.0 license. -

STELLAR SPECTRA A. Basic Line Formation
STELLAR SPECTRA A. Basic Line Formation R.J. Rutten Sterrekundig Instituut Utrecht February 6, 2007 Copyright c 1999 Robert J. Rutten, Sterrekundig Instuut Utrecht, The Netherlands. Copying permitted exclusively for non-commercial educational purposes. Contents Introduction 1 1 Spectral classification (“Annie Cannon”) 3 1.1 Stellar spectra morphology . 3 1.2 Data acquisition and spectral classification . 3 1.3 Introduction to IDL . 4 1.4 Introduction to LaTeX . 4 2 Saha-Boltzmann calibration of the Harvard sequence (“Cecilia Payne”) 7 2.1 Payne’s line strength diagram . 7 2.2 The Boltzmann and Saha laws . 8 2.3 Schadee’s tables for schadeenium . 12 2.4 Saha-Boltzmann populations of schadeenium . 14 2.5 Payne curves for schadeenium . 17 2.6 Discussion . 19 2.7 Saha-Boltzmann populations of hydrogen . 19 2.8 Solar Ca+ K versus Hα: line strength . 21 2.9 Solar Ca+ K versus Hα: temperature sensitivity . 24 2.10 Hot stars versus cool stars . 24 3 Fraunhofer line strengths and the curve of growth (“Marcel Minnaert”) 27 3.1 The Planck law . 27 3.2 Radiation through an isothermal layer . 29 3.3 Spectral lines from a solar reversing layer . 30 3.4 The equivalent width of spectral lines . 33 3.5 The curve of growth . 35 Epilogue 37 References 38 Text available at \tthttp://www.astro.uu.nl/~rutten/education/rjr-material/ssa (or via “Rob Rutten” in Google). Introduction These three exercises concern the appearance and nature of spectral lines in stellar spectra. Stellar spectrometry laid the foundation of astrophysics in the hands of: – Wollaston (1802): first observation of spectral lines in sunlight; – Fraunhofer (1814–1823): rediscovery of spectral lines in sunlight (“Fraunhofer lines”); their first systematic inventory. -

Spectral Classification and HR Diagram of Pre-Main Sequence Stars in NGC 6530
A&A 546, A9 (2012) Astronomy DOI: 10.1051/0004-6361/201219853 & c ESO 2012 Astrophysics Spectral classification and HR diagram of pre-main sequence stars in NGC 6530,, L. Prisinzano, G. Micela, S. Sciortino, L. Affer, and F. Damiani INAF – Osservatorio Astronomico di Palermo, Piazza del Parlamento, Italy 1, 90134 Palermo, Italy e-mail: [email protected] Received 20 June 2012 / Accepted 3 August 2012 ABSTRACT Context. Mechanisms involved in the star formation process and in particular the duration of the different phases of the cloud contrac- tion are not yet fully understood. Photometric data alone suggest that objects coexist in the young cluster NGC 6530 with ages from ∼1 Myr up to 10 Myr. Aims. We want to derive accurate stellar parameters and, in particular, stellar ages to be able to constrain a possible age spread in the star-forming region NGC 6530. Methods. We used low-resolution spectra taken with VLT/VIMOS and literature spectra of standard stars to derive spectral types of a subsample of 94 candidate members of this cluster. Results. We assign spectral types to 86 of the 88 confirmed cluster members and derive individual reddenings. Our data are better fitted by the anomalous reddening law with RV = 5. We confirm the presence of strong differential reddening in this region. We derive fundamental stellar parameters, such as effective temperatures, photospheric colors, luminosities, masses, and ages for 78 members, while for the remaining 8 YSOs we cannot determine the interstellar absorption, since they are likely accretors, and their V − I colors are bluer than their intrinsic colors. -

Opacities: Means & Uncertainties
OPACITIES: MEANS & Previously... UNCERTAINTIES Christopher Fontes Computational Physics Division Los Alamos National Laboratory ICTP-IAEA Advanced School and Workshop on Modern Methods in Plasma Spectroscopy Trieste, March 16-27, 2015 Operated by the Los Alamos National Security, LLC for the DOE/NNSA Slide 1 Before moving on to the topic of mean opacities, let’s look at Al opacities at different temperatures 19 -3 • Our main example is kT = 40 eV and Ne = 10 cm with <Z> = 10.05 (Li-like ions are dominant) • Consider raising and lowering the temperature: – kT = 400 eV (<Z> = 13.0; fully ionized) – kT = 20 eV (<Z> = 6.1; nitrogen-like stage is dominant) Slide 2 Slide 3 Slide 4 Slide 5 Slide 6 Slide 7 Road map to mean opacities Mean (gray) opacities In order of most to least refined • Under certain conditions, the need to transport a with respect to frequency resolution: frequency-dependent radiation intensity, Iν, can be relaxed in favor of an integrated intensity, I, given by ∞ κν (monochromatic) I = I dν ∫0 ν • Applying this notion of integrated quantities to each term of the radiation transport equation results in a new set of MG κ (multigroup) equations, similar to the original, frequency-dependent formulations • Frequency-dependent absorption terms that formerly (gray) contained will instead contain a suitably averaged κ κν “mean opacity” or “gray opacity” denoted by κ Slide 8 Slide 9 Mean opacities (continued) Types of mean opacities • The mean opacity κ represents, in a single number, the • Two most common types of gray opacities -

The Sun's Dynamic Atmosphere
Lecture 16 The Sun’s Dynamic Atmosphere Jiong Qiu, MSU Physics Department Guiding Questions 1. What is the temperature and density structure of the Sun’s atmosphere? Does the atmosphere cool off farther away from the Sun’s center? 2. What intrinsic properties of the Sun are reflected in the photospheric observations of limb darkening and granulation? 3. What are major observational signatures in the dynamic chromosphere? 4. What might cause the heating of the upper atmosphere? Can Sound waves heat the upper atmosphere of the Sun? 5. Where does the solar wind come from? 15.1 Introduction The Sun’s atmosphere is composed of three major layers, the photosphere, chromosphere, and corona. The different layers have different temperatures, densities, and distinctive features, and are observed at different wavelengths. Structure of the Sun 15.2 Photosphere The photosphere is the thin (~500 km) bottom layer in the Sun’s atmosphere, where the atmosphere is optically thin, so that photons make their way out and travel unimpeded. Ex.1: the mean free path of photons in the photosphere and the radiative zone. The photosphere is seen in visible light continuum (so- called white light). Observable features on the photosphere include: • Limb darkening: from the disk center to the limb, the brightness fades. • Sun spots: dark areas of magnetic field concentration in low-mid latitudes. • Granulation: convection cells appearing as light patches divided by dark boundaries. Q: does the full moon exhibit limb darkening? Limb Darkening: limb darkening phenomenon indicates that temperature decreases with altitude in the photosphere. Modeling the limb darkening profile tells us the structure of the stellar atmosphere. -

Radio Astronomy
Edition of 2013 HANDBOOK ON RADIO ASTRONOMY International Telecommunication Union Sales and Marketing Division Place des Nations *38650* CH-1211 Geneva 20 Switzerland Fax: +41 22 730 5194 Printed in Switzerland Tel.: +41 22 730 6141 Geneva, 2013 E-mail: [email protected] ISBN: 978-92-61-14481-4 Edition of 2013 Web: www.itu.int/publications Photo credit: ATCA David Smyth HANDBOOK ON RADIO ASTRONOMY Radiocommunication Bureau Handbook on Radio Astronomy Third Edition EDITION OF 2013 RADIOCOMMUNICATION BUREAU Cover photo: Six identical 22-m antennas make up CSIRO's Australia Telescope Compact Array, an earth-rotation synthesis telescope located at the Paul Wild Observatory. Credit: David Smyth. ITU 2013 All rights reserved. No part of this publication may be reproduced, by any means whatsoever, without the prior written permission of ITU. - iii - Introduction to the third edition by the Chairman of ITU-R Working Party 7D (Radio Astronomy) It is an honour and privilege to present the third edition of the Handbook – Radio Astronomy, and I do so with great pleasure. The Handbook is not intended as a source book on radio astronomy, but is concerned principally with those aspects of radio astronomy that are relevant to frequency coordination, that is, the management of radio spectrum usage in order to minimize interference between radiocommunication services. Radio astronomy does not involve the transmission of radiowaves in the frequency bands allocated for its operation, and cannot cause harmful interference to other services. On the other hand, the received cosmic signals are usually extremely weak, and transmissions of other services can interfere with such signals. -

SYNTHETIC SPECTRA for SUPERNOVAE Time a Basis for a Quantitative Interpretation. Eight Typical Spectra, Show
264 ASTRONOMY: PA YNE-GAPOSCHKIN AND WIIIPPLE PROC. N. A. S. sorption. I am indebted to Dr. Morgan and to Dr. Keenan for discussions of the problems of the calibration of spectroscopic luminosities. While these results are of a preliminary nature, it is apparent that spec- troscopic absolute magnitudes of the supergiants can be accurately cali- brated, even if few stars are available. For a calibration of a group of ten stars within Om5 a mean distance greater than one kiloparsec is necessary; for supergiants this requirement would be fulfilled for stars fainter than the sixth magnitude. The errors of the measured radial velocities need only be less than the velocity dispersion, that is, less than 8 km/sec. 1 Stebbins, Huffer and Whitford, Ap. J., 91, 20 (1940). 2 Greenstein, Ibid., 87, 151 (1938). 1 Stebbins, Huffer and Whitford, Ibid., 90, 459 (1939). 4Pub. Dom., Alp. Obs. Victoria, 5, 289 (1936). 5 Merrill, Sanford and Burwell, Ap. J., 86, 205 (1937). 6 Pub. Washburn Obs., 15, Part 5 (1934). 7Ap. J., 89, 271 (1939). 8 Van Rhijn, Gron. Pub., 47 (1936). 9 Adams, Joy, Humason and Brayton, Ap. J., 81, 187 (1935). 10 Merrill, Ibid., 81, 351 (1935). 11 Stars of High Luminosity, Appendix A (1930). SYNTHETIC SPECTRA FOR SUPERNOVAE By CECILIA PAYNE-GAPOSCHKIN AND FRED L. WHIPPLE HARVARD COLLEGE OBSERVATORY Communicated March 14, 1940 Introduction.-The excellent series of spectra of the supernovae in I. C. 4182 and N. G. C. 1003, published by Minkowski,I present for the first time a basis for a quantitative interpretation. Eight typical spectra, show- ing the major stages of the development during the first two hundred days, are shown in figure 1; they are directly reproduced from Minkowski's microphotometer tracings, with some smoothing for plate grain. -

Aerosol Optical Depth Value-Added Product
DOE/SC-ARM/TR-129 Aerosol Optical Depth Value-Added Product A Koontz C Flynn G Hodges J Michalsky J Barnard March 2013 DISCLAIMER This report was prepared as an account of work sponsored by the U.S. Government. Neither the United States nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the U.S. Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the U.S. Government or any agency thereof. DOE/SC-ARM/TR-129 Aerosol Optical Depth Value-Added Product A Koontz C Flynn G Hodges J Michalsky J Barnard March 2013 Work supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research A Koontz et al., March 2013, DOE/SC-ARM/TR-129 Contents 1.0 Introduction .......................................................................................................................................... 1 2.0 Description of Algorithm ...................................................................................................................... 1 2.1 Overview -

Chapter 19/ Optical Properties
Chapter 19 /Optical Properties The four notched and transpar- ent rods shown in this photograph demonstrate the phenomenon of photoelasticity. When elastically deformed, the optical properties (e.g., index of refraction) of a photoelastic specimen become anisotropic. Using a special optical system and polarized light, the stress distribution within the speci- men may be deduced from inter- ference fringes that are produced. These fringes within the four photoelastic specimens shown in the photograph indicate how the stress concentration and distribu- tion change with notch geometry for an axial tensile stress. (Photo- graph courtesy of Measurements Group, Inc., Raleigh, North Carolina.) Why Study the Optical Properties of Materials? When materials are exposed to electromagnetic radia- materials, we note that the performance of optical tion, it is sometimes important to be able to predict fibers is increased by introducing a gradual variation and alter their responses. This is possible when we are of the index of refraction (i.e., a graded index) at the familiar with their optical properties, and understand outer surface of the fiber. This is accomplished by the mechanisms responsible for their optical behaviors. the addition of specific impurities in controlled For example, in Section 19.14 on optical fiber concentrations. 766 Learning Objectives After careful study of this chapter you should be able to do the following: 1. Compute the energy of a photon given its fre- 5. Describe the mechanism of photon absorption quency and the value of Planck’s constant. for (a) high-purity insulators and semiconduc- 2. Briefly describe electronic polarization that re- tors, and (b) insulators and semiconductors that sults from electromagnetic radiation-atomic in- contain electrically active defects.