Gabapentin Background Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Métrologie De La Douleur Animale Sur Modèles Expérimentaux : Développement Et Validation De Biomarqueurs Neuroprotéomiques
Université de Montréal Métrologie de la douleur animale sur modèles expérimentaux : développement et validation de biomarqueurs neuroprotéomiques. par Colombe Otis Département de biomédecine vétérinaire Faculté de médecine vétérinaire Thèse présentée à la Faculté de médecine vétérinaire en vue de l’obtention du grade de philosophiæ doctor (Ph. D.) en sciences vétérinaires option pharmacologie Août, 2017 © Colombe Otis, 2017 Résumé La douleur est un phénomène complexe et dynamique comprenant un processus primaire et sensoriel, la nociception, auquel se rajoutent des réactions de défense et d'alarme psychophysiologiques. Ceci conduit au développement d'une véritable mémoire de la douleur, individuelle et modulée (plasticité neuronale) par des expériences précédentes de douleur, incluant entre autres, des phénomènes de sensibilisation nociceptive. Pour bien comprendre les mécanismes de sensibilisation centrale, les modèles animaux mimant les pathologies humaines sont d’une importance primordi ale. Or, pour ce faire, nous avons proposé une série d'approches expérimentales translationnelles par le développement de modèles expérimentaux de douleur bovine, canine et murine afin de caractériser les atteintes du protéome spinal en fonction du niveau de douleur perçu par l'animal et d’identifier le (les) marqueur (s) potentiellement modulé (s) par la sensibilisation nociceptive. À l’aide de la neuroprotéomique, nous avons identifié sur un modèle bovin de douleur viscérale, la protéine transthyrétine (TTR) comme étant sous-exprimée dans le protéome spinal en présence de douleur et ce, vérifié également dans le modèle canin de douleur chronique liée à l’arthrose chirurgicale. Ces résultats suggèrent la possibilité d’entrevoir la TTR comme un biomarqueur potentiel de la douleur chronique. Notre découverte de la relation entre les niveaux spinaux de TTR par neuroprotéomique et l’ hypersensibilité à la douleur dans les modèles bovin et canin soutiennent l’implication de composantes inflammatoires nociceptives et immunitaires. -

Big Pain Assays Aren't a Big Pain with the Raptor Biphenyl LC Column
Featured Application: 231 Pain Management and Drugs of Abuse Compounds in under 10 Minutes by LC-MS/MS Big Pain Assays Aren’t a Big Pain with the Raptor Biphenyl LC Column • 231 compounds, 40+ isobars, 10 drug classes, 22 ESI- compounds in 10 minutes with 1 column. • A Raptor SPP LC column with time-tested Restek Biphenyl selectivity is the most versatile, multiclass-capable LC column available. • Achieve excellent separation of critical isobars with no tailing peaks. • Run fast and reliable high-throughput LC-MS/MS analyses with increased sensitivity using simple mobile phases. The use of pain management drugs is steadily increasing. As a result, hospital and reference labs are seeing an increase in patient samples that must be screened for a wide variety of pain management drugs to prevent drug abuse and to ensure patient safety and adherence to their medication regimen. Thera- peutic drug monitoring can be challenging due to the low cutoff levels, potential matrix interferences, and isobaric drug compounds. To address these chal- lenges, many drug testing facilities are turning to liquid chromatography coupled with mass spectrometry (LC-MS/MS) for its increased speed, sensitivity, and specificity. As shown in the analysis below, Restek’s Raptor Biphenyl column is ideal for developing successful LC-MS/MS pain medication screening methodologies. With its exceptionally high retention and unique selectivity, 231 multiclass drug compounds and metabolites—including over 40 isobars—can be analyzed in just 10 minutes. In addition, separate panels have been optimized on the Raptor Biphenyl column specifically for opioids, antianxiety drugs, barbiturates, NSAIDs and analgesics, antidepressants, antiepileptics, antipsychotics, hallucinogens, and stimulants for use during confirmation and quantitative analyses. -

A Bill to Repeal Criminal Drug Laws: Replacing Prohibition with Regulation Joseph L
Hofstra Law Review Volume 18 | Issue 3 Article 10 1990 A Bill to Repeal Criminal Drug Laws: Replacing Prohibition with Regulation Joseph L. Galiber Follow this and additional works at: http://scholarlycommons.law.hofstra.edu/hlr Part of the Law Commons Recommended Citation Galiber, Joseph L. (1990) "A Bill to Repeal Criminal Drug Laws: Replacing Prohibition with Regulation," Hofstra Law Review: Vol. 18: Iss. 3, Article 10. Available at: http://scholarlycommons.law.hofstra.edu/hlr/vol18/iss3/10 This document is brought to you for free and open access by Scholarly Commons at Hofstra Law. It has been accepted for inclusion in Hofstra Law Review by an authorized administrator of Scholarly Commons at Hofstra Law. For more information, please contact [email protected]. Galiber: A Bill to Repeal Criminal Drug Laws: Replacing Prohibition with R A BILL TO REPEAL CRIMINAL DRUG LAWS: REPLACING PROHIBITION WITH REGULATION Joseph L. Galiber* Conventional wisdom obliges elected officials to beat the narcodrums loudly and incessantly, and to demand increasingly harsh criminal penalties for the sale and use of illegal drugs.' It is reasonable to wonder why I, a senator, would dare submit a bill2 to the New York State Legislature which would regulate all drugs cur- rently proscribed as illegal in precisely the same manner as alcohol.3 The short answer is that the use of the criminal law to control drug use has not, and never will, have anything more than a costly and marginal impact on drug consumption.4 Despite all the public hyperventilation, drug consumption remains a private, consensual * New York State Senator; B.A. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Definition of Controlled Substance Schedules
UPDATED MARCH 2018 DEFINITION OF CONTROLLED SUBSTANCE SCHEDULES Drugs and other substances that are considered controlled substances under the Controlled Substances Act (CSA) are divided into five schedules. An updated and complete list of the schedules is published annually in Title 21 Code of Federal Regulations (C.F.R.) §§ 1308.11 through 1308.15. Substances are placed in their respective schedules based on whether they have a currently accepted medical use in treatment in the U.S., their relative abuse potential, and likelihood of causing dependence when abused. Examples of the drugs in each schedule are listed below. Schedule I Controlled Substances Substances in this schedule have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. Some examples of substances listed in Schedule I are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), peyote, methaqualone, and 3,4- methylenedioxymethamphetamine ("Ecstasy"). Schedule II/IIN Controlled Substances (2/2N) Substances in this schedule have a high potential for abuse which may lead to severe psychological or physical dependence. Examples of Schedule II narcotics include: hydromorphone (Dilaudid®), methadone (Dolophine®), meperidine (Demerol®), oxycodone (OxyContin®, Percocet®), and fentanyl (Sublimaze®, Duragesic®). Other Schedule II narcotics include: morphine, opium, codeine, and hydrocodone. Examples of Schedule IIN stimulants include: amphetamine (Dexedrine®, Adderall®), methamphetamine (Desoxyn®), and methylphenidate (Ritalin®). Other Schedule II substances include: amobarbital, glutethimide, and pentobarbital. 1 Schedule III/IIIN Controlled Substances (3/3N) Substances in this schedule have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence. -

Synthetic Drugs: Overview and Issues for Congress
Synthetic Drugs: Overview and Issues for Congress Lisa N. Sacco Analyst in Illicit Drugs and Crime Policy Kristin Finklea Specialist in Domestic Security May 3, 2016 Congressional Research Service 7-5700 www.crs.gov R42066 Synthetic Drugs: Overview and Issues for Congress Summary Synthetic drugs, as opposed to natural drugs, are chemically produced in a laboratory. Their chemical structure can be either identical to or different from naturally occurring drugs, and their effects are designed to mimic or even enhance those of natural drugs. When produced clandestinely, they are not typically controlled pharmaceutical substances intended for legitimate medical use. Designer drugs are a form of synthetic drugs. They contain slightly modified molecular structures of illegal or controlled substances, and they are modified in order to circumvent existing drug laws. While the issue of synthetic drugs and their abuse is not new, Congress has demonstrated a renewed concern with the issue. From 2009 to 2011, synthetic drug abuse was reported to have dramatically increased. During this time period, calls to poison control centers for incidents relating to harmful effects of synthetic cannabinoids (such as “K2” and “Spice”) and stimulants (such as “bath salts”) increased at what some considered to be an alarming rate. The number of hospital emergency department visits involving synthetic cannabinoids more than doubled from 2010 to 2011. In 2012 and 2013, however, the number of calls to poison control centers for incidents relating to harmful effects of synthetic cannabinoids and synthetic stimulants decreased. Calls regarding bath salts have declined each year since 2011, while calls regarding synthetic cannabinoids have increased since the drops in 2012 and 2013. -
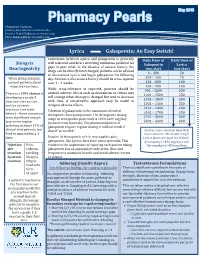
Lyrica Gabapentin: an Easy Switch!
Pharmacist Contacts: [email protected]; [email protected]; [email protected] Lyrica Gabapentin: An Easy Switch! Conversion between Lyrica and gabapentin is generally Daily Dose of Daily Dose of Shingrix well tolerated and direct switching minimizes potential for Gabapentin Lyrica Reactogenicity gaps in pain relief. In the absence of seizure history, the (mg/day) (mg/day) drugs can be directly interchanged; patients can be advised 0 – 300 50 to discontinue Lyrica and begin gabapentin the following When giving Shingrix, day. Patients with a seizure history should be cross-tapered 301 – 450 75 counsel patients about over 1 – 4 weeks. 451 – 600 100 expected reactions. 601 – 900 150 While cross-tolerance is expected, patients should be 901 – 1200 200 advised adverse effects such as drowsiness or edema may There is a 10% chance of 1201 – 1500 250 still emerge when therapy is changed but tend to decrease developing a grade 3 1501 – 1800 300 injection site reaction with time. A conservative approach may be useful to 1801 – 2100 350 and/or systemic mitigate adverse effects. reactions (see table 2101 – 2400 400 Titration of gabapentin to the maximum tolerated 2401 – 2700 450 below) – these symptoms therapeutic dose is important. The therapeutic dosing 2701 – 3000 500 were significant enough range in neuropathic pain trials is 1800-3600 mg/day to prevent regular (normal renal function). The pharmacokinetics of 3001 – 3600 600 activities in about 17% of gabapentin require regular dosing, it will not work if clinical trial patients, but dosed “as needed.” Studies show minimal benefit & tend to pass within 2-3 more adverse effects when high days. -

House of Representatives Staff Analysis Bill #: Hb 6095
HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: HB 6095 Scheduling of Drug Products Containing Cannabidiol SPONSOR(S): Fischer TIED BILLS: IDEN./SIM. BILLS: SB 1476 REFERENCE ACTION ANALYST STAFF DIRECTOR or BUDGET/POLICY CHIEF 1) Professions & Public Health Subcommittee 17 Y, 0 N Morris McElroy 2) Criminal Justice & Public Safety Subcommittee 18 Y, 0 N Padgett Hall 3) Health & Human Services Committee 20 Y, 0 N Morris Calamas SUMMARY ANALYSIS Federal and state law both classify controlled substances into five schedules. The scheduling determination for a controlled substance is based on a substance’s potential for abuse, accepted medical use, and potential for addiction. The classifications range from a Schedule I substance, which has a high potential for abuse, with no accepted medical use, and high potential for addiction; to a Schedule V substance, which has a low potential for abuse, an accepted medical use, and a mild potential for addiction. Generally, cannabis and compounds derived from cannabis are listed in Schedule I of both federal and Florida law. Cannabis contains delta-9-tetrahydrocannabinol (THC), which is the psychoactive chemical in marijuana which produces the “high” commonly associated with marijuana use. Epidiolex is a prescription cannabidiol, a non-psychoactive compound derived from the cannabis plant which is used to treat seizures. Epidiolex does not contain THC. On June 25, 2018, the U.S. Food and Drug Administration approved Epidiolex for use by patients two years of age or older and the federal Drug Enforcement Administration (DEA) rescheduled Epidiolex in Schedule V of the federal Controlled Substances Act (CSA). In 2019, the Legislature, mirroring federal law, formally rescheduled Epidiolex as a Schedule V controlled substance. -

Comparing Adverse Effects of Analgesic Strategies For
Comparing Adverse Effects daily and weekly time during which higher than that of the only ATC opi- their pain interfered with their mood oids group and 17 times higher than of Analgesic Strategies for or activities, and they indicated the that of the only PRN opioids group. Chronic Cancer Pain amount of relief they received from Source: J Pain Symptom Manage. 2007;33(1):67–77. their pain medicine in the previ- Nausea, vomiting, drowsiness, lack ous week. They also completed the of energy, urinary retention—some- Karnofsky Performance Status (KPS), Clopidogrel: Best Results times the adverse effects of the anal- which measures patients’ ability to per- Pre- or Post-PCI? gesic medication are enough to derail form activities of daily living and their pain management for patients with need for caregiver assistance. When is the best time to give the anti- cancer. It would help to understand There were no significant differ- platelet clopidogrel to patients sched- the relationships between the type of ences between any of the four groups uled for diagnostic coronary angiogra- analgesic prescription and the preva- in terms of pain intensity or amount of phy—before ad hoc coronary stenting lence and severity of adverse effects. time spent in pain. Total pain interfer- or immediately after? Researchers from But not much information is available, ence scores, however, were significantly University of Debrecen, Debrecen, say researchers from the University higher in the ATC plus PRN opioids Hungary and Medical University of of California, San Francisco; the Uni- group than in the no opioids group. Vienna and Wilhelminenhospital, both versity of Nebraska, Omaha; and the The ATC plus PRN opioids group in Vienna, Austria say starting treat- University of Texas Southwestern Med- also had significantly lower functional ment before percutaneous coronary ical Center, Dallas. -

Gabapentin Abuse Gabapentin (Neurontin®) Is Indicated for the Treatment of Epilepsy and Neuro- Pathic Pain, Including Post-Herpetic Neuralgia
November 2018 Poison Center Hotline: 1-800-222-1222 The Maryland Poison Center’s Monthly Update: News, Advances, Information Gabapentin Abuse Gabapentin (Neurontin®) is indicated for the treatment of epilepsy and neuro- pathic pain, including post-herpetic neuralgia. Gabapentin is often prescribed off -label for a variety of conditions including insomnia, anxiety, bipolar disorder, migraines, drug and alcohol addiction, and others. Off-label use exceeds use for FDA approved indications. Termed a gabapentinoid, it is an analog of γ- aminobutyric acid (GABA) that increases GABA but does not bind to GABA re- ceptors. It has a selective inhibitory effect on voltage-gated calcium channels containing the α2δ1 subunit. Its exact mechanism for epilepsy and pain is un- clear. Gabapentin is not a controlled substance as it was believed that gabapentin had no abuse potential when approved by the FDA in 1993. Overall, if used at thera- Did you know? peutic doses by patients without a substance abuse/misuse history, the risk of misuse is probably lower than that of other drugs such as benzodiazepines. There are reports of abuse of However, reports are increasing of gabapentin misuse and abuse. Motivations pregabalin (Lyrica ®), another for misuse include recreational use, mood and/or anxiety control, potentiating gabapentinoid used for the effects of drug abuse treatment, pain management, reducing cravings for neuropathic pain, post-herpetic other drugs, managing withdrawal from other drugs, substituting for other pain and fibromyalgia. drugs, and gabapentin addiction (Addiction 2016;11:1160-74). The risk of misuse Approved by the FDA in 2004, increases in people with a history of recreational drug abuse who take gabapen- pregabalin is Schedule V. -

Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review
Current Pain and Headache Reports (2019) 23: 37 https://doi.org/10.1007/s11916-019-0774-0 OTHER PAIN (A KAYE AND N VADIVELU, SECTION EDITORS) Membrane Stabilizer Medications in the Treatment of Chronic Neuropathic Pain: a Comprehensive Review Omar Viswanath1,2,3 & Ivan Urits4 & Mark R. Jones4 & Jacqueline M. Peck5 & Justin Kochanski6 & Morgan Hasegawa6 & Best Anyama7 & Alan D. Kaye7 Published online: 1 May 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Neuropathic pain is often debilitating, severely limiting the daily lives of patients who are affected. Typically, neuropathic pain is difficult to manage and, as a result, leads to progression into a chronic condition that is, in many instances, refractory to medical management. Recent Findings Gabapentinoids, belonging to the calcium channel blocking class of drugs, have shown good efficacy in the management of chronic pain and are thus commonly utilized as first-line therapy. Various sodium channel blocking drugs, belonging to the categories of anticonvulsants and local anesthetics, have demonstrated varying degrees of efficacy in the in the treatment of neurogenic pain. Summary Though there is limited medical literature as to efficacy of any one drug, individualized multimodal therapy can provide significant analgesia to patients with chronic neuropathic pain. Keywords Neuropathic pain . Chronic pain . Ion Channel blockers . Anticonvulsants . Membrane stabilizers Introduction Neuropathic pain, which is a result of nervous system injury or lives of patients who are affected. Frequently, it is difficult to dysfunction, is often debilitating, severely limiting the daily manage and as a result leads to the progression of a chronic condition that is, in many instances, refractory to medical This article is part of the Topical Collection on Other Pain management.