Additive and Interactive Effects of Plant Genotypic Diversity on Arthropod Communities and Plant fitness
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Untangling Taxonomy: a DNA Barcode Reference Library for Canadian Spiders
Molecular Ecology Resources (2016) 16, 325–341 doi: 10.1111/1755-0998.12444 Untangling taxonomy: a DNA barcode reference library for Canadian spiders GERGIN A. BLAGOEV, JEREMY R. DEWAARD, SUJEEVAN RATNASINGHAM, STEPHANIE L. DEWAARD, LIUQIONG LU, JAMES ROBERTSON, ANGELA C. TELFER and PAUL D. N. HEBERT Biodiversity Institute of Ontario, University of Guelph, Guelph, ON, Canada Abstract Approximately 1460 species of spiders have been reported from Canada, 3% of the global fauna. This study provides a DNA barcode reference library for 1018 of these species based upon the analysis of more than 30 000 specimens. The sequence results show a clear barcode gap in most cases with a mean intraspecific divergence of 0.78% vs. a min- imum nearest-neighbour (NN) distance averaging 7.85%. The sequences were assigned to 1359 Barcode index num- bers (BINs) with 1344 of these BINs composed of specimens belonging to a single currently recognized species. There was a perfect correspondence between BIN membership and a known species in 795 cases, while another 197 species were assigned to two or more BINs (556 in total). A few other species (26) were involved in BIN merges or in a combination of merges and splits. There was only a weak relationship between the number of specimens analysed for a species and its BIN count. However, three species were clear outliers with their specimens being placed in 11– 22 BINs. Although all BIN splits need further study to clarify the taxonomic status of the entities involved, DNA bar- codes discriminated 98% of the 1018 species. The present survey conservatively revealed 16 species new to science, 52 species new to Canada and major range extensions for 426 species. -

The Pentatomidae, Or Stink Bugs, of Kansas with a Key to Species (Hemiptera: Heteroptera) Richard J
Fort Hays State University FHSU Scholars Repository Biology Faculty Papers Biology 2012 The eP ntatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera) Richard J. Packauskas Fort Hays State University, [email protected] Follow this and additional works at: http://scholars.fhsu.edu/biology_facpubs Part of the Biology Commons, and the Entomology Commons Recommended Citation Packauskas, Richard J., "The eP ntatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera)" (2012). Biology Faculty Papers. 2. http://scholars.fhsu.edu/biology_facpubs/2 This Article is brought to you for free and open access by the Biology at FHSU Scholars Repository. It has been accepted for inclusion in Biology Faculty Papers by an authorized administrator of FHSU Scholars Repository. 210 THE GREAT LAKES ENTOMOLOGIST Vol. 45, Nos. 3 - 4 The Pentatomidae, or Stink Bugs, of Kansas with a key to species (Hemiptera: Heteroptera) Richard J. Packauskas1 Abstract Forty eight species of Pentatomidae are listed as occurring in the state of Kansas, nine of these are new state records. A key to all species known from the state of Kansas is given, along with some notes on new state records. ____________________ The family Pentatomidae, comprised of mainly phytophagous and a few predaceous species, is one of the largest families of Heteroptera. Some of the phytophagous species have a wide host range and this ability may make them the most economically important family among the Heteroptera (Panizzi et al. 2000). As a group, they have been found feeding on cotton, nuts, fruits, veg- etables, legumes, and grain crops (McPherson 1982, McPherson and McPherson 2000, Panizzi et al 2000). -

Of Washington State
The Great Lakes Entomologist Volume 45 Numbers 3 & 4 - Fall/Winter 2012 Numbers 3 & Article 11 4 - Fall/Winter 2012 October 2012 The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Washington State Richard S. Zack Washington State University Peter J. Landolt USDA Joseph E. Munyaneza USDA Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Zack, Richard S.; Landolt, Peter J.; and Munyaneza, Joseph E. 2012. "The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Washington State," The Great Lakes Entomologist, vol 45 (2) Available at: https://scholar.valpo.edu/tgle/vol45/iss2/11 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Zack et al.: The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Washingt 2012 THE GREAT LAKES ENTOMOLOGIST 251 The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Washington State Richard S. Zack1, Peter J. Landolt2 and Joseph E. Munyaneza2 Abstract Froeschner (1988) recorded 23 species of stink bugs (Hemiptera: Heterop- tera: Pentatomidae) from Washington State. Based on material primarily housed in the M. T. James Entomological Collection at Washington State University, the number of species is increased to 51. Three species recorded by Froeschner (1988) were not found in our collections: Apateticus crocatus (Uhler), Chloro- chroa rossiana Buxton and Thomas, and Tepa rugulosa (Say). Species recorded from Washington State for the first time are: Apoecilus bracteatus (Fitch), Perillus bioculatus (Fabricius), Podisus maculiventris (Say), P. -
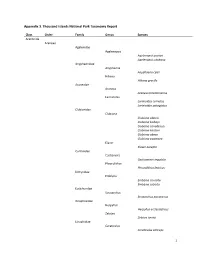
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

Molecular Insights Into the Phylogenetic Structure of the Spider
MolecularBlackwell Publishing Ltd insights into the phylogenetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna MIQUEL A. ARNEDO, INGI AGNARSSON & ROSEMARY G. GILLESPIE Accepted: 9 March 2007 Arnedo, M. A., Agnarsson, I. & Gillespie, R. G. (2007). Molecular insights into the phylo- doi:10.1111/j.1463-6409.2007.00280.x genetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna. — Zoologica Scripta, 36, 337–352. The Hawaiian happy face spider (Theridion grallator Simon, 1900), named for a remarkable abdominal colour pattern resembling a smiling face, has served as a model organism for under- standing the generation of genetic diversity. Theridion grallator is one of 11 endemic Hawaiian species of the genus reported to date. Asserting the origin of island endemics informs on the evolutionary context of diversification, and how diversity has arisen on the islands. Studies on the genus Theridion in Hawaii, as elsewhere, have long been hampered by its large size (> 600 species) and poor definition. Here we report results of phylogenetic analyses based on DNA sequences of five genes conducted on five diverse species of Hawaiian Theridion, along with the most intensive sampling of Theridiinae analysed to date. Results indicate that the Hawai- ian Islands were colonised by two independent Theridiinae lineages, one of which originated in the Americas. Both lineages have undergone local diversification in the archipelago and have convergently evolved similar bizarre morphs. Our findings confirm para- or polyphyletic status of the largest Theridiinae genera: Theridion, Achaearanea and Chrysso. -

The Life History and Management of Phyllotreta Cruciferae and Phyllotreta Striolata (Coleoptera: Chrysomelidae), Pests of Brassicas in the Northeastern United States
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 2004 The life history and management of Phyllotreta cruciferae and Phyllotreta striolata (Coleoptera: Chrysomelidae), pests of brassicas in the northeastern United States. Caryn L. Andersen University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Andersen, Caryn L., "The life history and management of Phyllotreta cruciferae and Phyllotreta striolata (Coleoptera: Chrysomelidae), pests of brassicas in the northeastern United States." (2004). Masters Theses 1911 - February 2014. 3091. Retrieved from https://scholarworks.umass.edu/theses/3091 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. THE LIFE HISTORY AND MANAGEMENT OF PHYLLOTRETA CRUCIFERAE AND PHYLLOTRETA STRIOLATA (COLEOPTERA: CHRYSOMELIDAE), PESTS OF BRASSICAS IN THE NORTHEASTERN UNITED STATES A Thesis Presented by CARYN L. ANDERSEN Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE September 2004 Entomology © Copyright by Caryn L. Andersen 2004 All Rights Reserved THE LIFE HISTORY AND MANAGEMENT OF PHYLLOTRETA CRUCIFERAE AND PHYLLOTRETA STRIOLATA (COLEOPTERA: CHRYSOMELIDAE), PESTS OF BRASSICAS IN THE NORTHEASTERN UNITED STATES A Thesis Presented by CARYN L. ANDERSEN Approved as to style and content by: Tt, Francis X. Mangan, Member Plant, Soil, and Insect Sciences DEDICATION To my family and friends. ACKNOWLEDGMENTS I would like to thank my advisors, Roy Van Driesche and Ruth Hazzard, for their continual support, encouragement and thoughtful advice. -

Companion Planting and Insect Pest Control
Chapter 1 Companion Planting and Insect Pest Control Joyce E. Parker, William E. Snyder, George C. Hamilton and Cesar Rodriguez‐Saona Additional information is available at the end of the chapter http://dx.doi.org/10.5772/55044 1. Introduction There is growing public concern about pesticides’ non-target effects on humans and other organisms, and many pests have evolved resistance to some of the most commonly-used pesticides. Together, these factors have led to increasing interest in non-chemical, ecologically- sound ways to manage pests [1]. One pest-management alternative is the diversification of agricultural fields by establishing “polycultures” that include one or more different crop varieties or species within the same field, to more-closely match the higher species richness typical of natural systems [2, 3]. After all, destructive, explosive herbivore outbreaks typical of agricultural monocultures are rarely seen in highly-diverse unmanaged communities. There are several reasons that diverse plantings might experience fewer pest problems. First, it can be more difficult for specialized herbivores to “find” their host plant against a back‐ ground of one or more non-host species [4]. Second, diverse plantings may provide a broader base of resources for natural enemies to exploit, both in terms of non-pest prey species and resources such as pollen and nectar provided by the plant themselves, building natural enemy communities and strengthening their impacts on pests [4]. Both host-hiding and encourage‐ ment of natural enemies have the potential to depress pest populations, reducing the need for pesticide applications and increasing crop yields [5, 6]. On the other hand, crop diversification can present management and economic challenges for farmers, making these schemes difficult to implement. -

A Protocol for Online Documentation of Spider Biodiversity Inventories Applied to a Mexican Tropical Wet Forest (Araneae, Araneomorphae)
Zootaxa 4722 (3): 241–269 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4722.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:6AC6E70B-6E6A-4D46-9C8A-2260B929E471 A protocol for online documentation of spider biodiversity inventories applied to a Mexican tropical wet forest (Araneae, Araneomorphae) FERNANDO ÁLVAREZ-PADILLA1, 2, M. ANTONIO GALÁN-SÁNCHEZ1 & F. JAVIER SALGUEIRO- SEPÚLVEDA1 1Laboratorio de Aracnología, Facultad de Ciencias, Departamento de Biología Comparada, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Colonia Copilco el Bajo. C. P. 04510. Del. Coyoacán, Ciudad de México, México. E-mail: [email protected] 2Corresponding author Abstract Spider community inventories have relatively well-established standardized collecting protocols. Such protocols set rules for the orderly acquisition of samples to estimate community parameters and to establish comparisons between areas. These methods have been tested worldwide, providing useful data for inventory planning and optimal sampling allocation efforts. The taxonomic counterpart of biodiversity inventories has received considerably less attention. Species lists and their relative abundances are the only link between the community parameters resulting from a biotic inventory and the biology of the species that live there. However, this connection is lost or speculative at best for species only partially identified (e. g., to genus but not to species). This link is particularly important for diverse tropical regions were many taxa are undescribed or little known such as spiders. One approach to this problem has been the development of biodiversity inventory websites that document the morphology of the species with digital images organized as standard views. -

Spider Biodiversity Patterns and Their Conservation in the Azorean
Systematics and Biodiversity 6 (2): 249–282 Issued 6 June 2008 doi:10.1017/S1477200008002648 Printed in the United Kingdom C The Natural History Museum ∗ Paulo A.V. Borges1 & Joerg Wunderlich2 Spider biodiversity patterns and their 1Azorean Biodiversity Group, Departamento de Ciˆencias conservation in the Azorean archipelago, Agr´arias, CITA-A, Universidade dos Ac¸ores. Campus de Angra, with descriptions of new species Terra-Ch˜a; Angra do Hero´ısmo – 9700-851 – Terceira (Ac¸ores); Portugal. Email: [email protected] 2Oberer H¨auselbergweg 24, Abstract In this contribution, we report on patterns of spider species diversity of 69493 Hirschberg, Germany. the Azores, based on recently standardised sampling protocols in different hab- Email: joergwunderlich@ t-online.de itats of this geologically young and isolated volcanic archipelago. A total of 122 species is investigated, including eight new species, eight new records for the submitted December 2005 Azorean islands and 61 previously known species, with 131 new records for indi- accepted November 2006 vidual islands. Biodiversity patterns are investigated, namely patterns of range size distribution for endemics and non-endemics, habitat distribution patterns, island similarity in species composition and the estimation of species richness for the Azores. Newly described species are: Oonopidae – Orchestina furcillata Wunderlich; Linyphiidae: Linyphiinae – Porrhomma borgesi Wunderlich; Turinyphia cavernicola Wunderlich; Linyphiidae: Micronetinae – Agyneta depigmentata Wunderlich; Linyph- iidae: -

Identification, Biology, Impacts, and Management of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Soybean and Corn in the Midwestern United States
Journal of Integrated Pest Management (2017) 8(1):11; 1–14 doi: 10.1093/jipm/pmx004 Profile Identification, Biology, Impacts, and Management of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Soybean and Corn in the Midwestern United States Robert L. Koch,1,2 Daniela T. Pezzini,1 Andrew P. Michel,3 and Thomas E. Hunt4 1 Department of Entomology, University of Minnesota, 1980 Folwell Ave., Saint Paul, MN 55108 ([email protected]; Downloaded from https://academic.oup.com/jipm/article-abstract/8/1/11/3745633 by guest on 08 January 2019 [email protected]), 2Corresponding author, e-mail: [email protected], 3Department of Entomology, Ohio Agricultural Research and Development Center, The Ohio State University, 210 Thorne, 1680 Madison Ave. Wooster, OH 44691 ([email protected]), and 4Department of Entomology, University of Nebraska, Haskell Agricultural Laboratory, 57905 866 Rd., Concord, NE 68728 ([email protected]) Subject Editor: Jeffrey Davis Received 12 December 2016; Editorial decision 22 March 2017 Abstract Stink bugs (Hemiptera: Heteroptera: Pentatomidae) are an emerging threat to soybean and corn production in the midwestern United States. An invasive species, the brown marmorated stink bug, Halyomorpha halys (Sta˚ l), is spreading through the region. However, little is known about the complex of stink bug species associ- ated with corn and soybean in the midwestern United States. In this region, particularly in the more northern states, stink bugs have historically caused only infrequent impacts to these crops. To prepare growers and agri- cultural professionals to contend with this new threat, we provide a review of stink bugs associated with soybean and corn in the midwestern United States.