Pesticidally Active Heterocyclic Derivatives with Sulphur Containing Substituents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Integration of Entomopathogenic Fungi Into IPM Programs: Studies Involving Weevils (Coleoptera: Curculionoidea) Affecting Horticultural Crops
insects Review Integration of Entomopathogenic Fungi into IPM Programs: Studies Involving Weevils (Coleoptera: Curculionoidea) Affecting Horticultural Crops Kim Khuy Khun 1,2,* , Bree A. L. Wilson 2, Mark M. Stevens 3,4, Ruth K. Huwer 5 and Gavin J. Ash 2 1 Faculty of Agronomy, Royal University of Agriculture, P.O. Box 2696, Dangkor District, Phnom Penh, Cambodia 2 Centre for Crop Health, Institute for Life Sciences and the Environment, University of Southern Queensland, Toowoomba, Queensland 4350, Australia; [email protected] (B.A.L.W.); [email protected] (G.J.A.) 3 NSW Department of Primary Industries, Yanco Agricultural Institute, Yanco, New South Wales 2703, Australia; [email protected] 4 Graham Centre for Agricultural Innovation (NSW Department of Primary Industries and Charles Sturt University), Wagga Wagga, New South Wales 2650, Australia 5 NSW Department of Primary Industries, Wollongbar Primary Industries Institute, Wollongbar, New South Wales 2477, Australia; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +61-46-9731208 Received: 7 September 2020; Accepted: 21 September 2020; Published: 25 September 2020 Simple Summary: Horticultural crops are vulnerable to attack by many different weevil species. Fungal entomopathogens provide an attractive alternative to synthetic insecticides for weevil control because they pose a lesser risk to human health and the environment. This review summarises the available data on the performance of these entomopathogens when used against weevils in horticultural crops. We integrate these data with information on weevil biology, grouping species based on how their developmental stages utilise habitats in or on their hostplants, or in the soil. -

Distribution of Methionine Sulfoxide Reductases in Fungi and Conservation of the Free- 2 Methionine-R-Sulfoxide Reductase in Multicellular Eukaryotes
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.26.433065; this version posted February 27, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Distribution of methionine sulfoxide reductases in fungi and conservation of the free- 2 methionine-R-sulfoxide reductase in multicellular eukaryotes 3 4 Hayat Hage1, Marie-Noëlle Rosso1, Lionel Tarrago1,* 5 6 From: 1Biodiversité et Biotechnologie Fongiques, UMR1163, INRAE, Aix Marseille Université, 7 Marseille, France. 8 *Correspondence: Lionel Tarrago ([email protected]) 9 10 Running title: Methionine sulfoxide reductases in fungi 11 12 Keywords: fungi, genome, horizontal gene transfer, methionine sulfoxide, methionine sulfoxide 13 reductase, protein oxidation, thiol oxidoreductase. 14 15 Highlights: 16 • Free and protein-bound methionine can be oxidized into methionine sulfoxide (MetO). 17 • Methionine sulfoxide reductases (Msr) reduce MetO in most organisms. 18 • Sequence characterization and phylogenomics revealed strong conservation of Msr in fungi. 19 • fRMsr is widely conserved in unicellular and multicellular fungi. 20 • Some msr genes were acquired from bacteria via horizontal gene transfers. 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.26.433065; this version posted February 27, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

The Effectiveness of a Pyriprole \(125 Mg/Ml\) and a Metaflumizone \(150
Article available at http://www.parasite-journal.org or http://dx.doi.org/10.1051/parasite/2008151093 THE EFFECTIVENESS OF A PYRIPROLE (125 MG/ML) AND A METAFLUMIZONE (150 MG/ML) COMBINED WITH AMITRAZ (150 MG/ML) SPOT-ON TREATMENT IN PREVENTING PHLEBOTOMUS PERNICIOSUS FROM FEEDING ON DOGS THOMAS C.*, ROQUES M.* & FRANC M.* Summary: Résumé : PYRIPROLE ET ASSOCIATION MÉTAFLUMIZONE-AMITRAZ : ÉTUDE DE L’ACTIVITÉ ANTI-GORGEMENT VIS-À-VIS DE PHLEBOTOMUS A controlled clinical trial was performed to assess the effectiveness PERNICIOSUS SUR LE CHIEN TRAITÉ PAR CES FORMULATIONS SPOT-ON of a pyriprole (125 mg/ml) and a metaflumizone (150 mg/ml) combined with amitraz (150 mg/ml) spot-on treatment Cet essai avait pour but d’étudier l’efficacité de deux spot-on (recommended dosage) in preventing adult female sandflies destinés au chien – pyriprole (125 mg/ml) et métaflumizone (Phlebotomus perniciosus) from feeding on dogs. Sandfly mortality (150 mg/ml) associée à l’amitraz (150 mg/ml) – sur les was also assessed. Twelve beagle dogs were used in the study. phlébotomes (effet létal et effet antigorgement). 12 chiens ont été Prior to treatment they were checked for their attractiveness to sand- utilisés. Ils ont été répartis en trois lots de quatre en fonction de flies, ranked accordingly to generate partner triplets of equivalent leur attractivité pour les femelles de phlébotomes. Un lot a été sensitivity to sandflies: four control dogs, four treated with the traité avec le spot-on au pyriprole, un lot avec la métaflumizone pyriprole and four with the metaflumizone spot-on. The dogs were associée à de l’amitraz, le dernier lot étant le lot témoin non challenged with 50 unfed adult female sandflies (8-10 days old), traité. -

Efficacy of a Fixed Combination of Permethrin 54.5% and Fipronil 6.1
Bonneau et al. Parasites & Vectors (2015) 8:204 DOI 10.1186/s13071-015-0805-6 RESEARCH Open Access Efficacy of a fixed combination of permethrin 54.5% and fipronil 6.1% (Effitix®) in dogs experimentally infested with Ixodes ricinus Stéphane Bonneau1†, Nadège Reymond2†, Sandeep Gupta3 and Christelle Navarro1* Abstract Background: Ticks are the most important vectors of disease-causing pathogens in domestic animals and are considered to be second worldwide to mosquitoes as vectors of human diseases. In Europe, Ixodes ricinus,the sheep tick, plays an important role as companion animal parasite but is also the primary vector of medically important diseases such as tick-borne encephalitis and Lyme borreliosis. The present study was designed to evaluate the efficacy under laboratory conditions of a new fixed spot-on combination of fipronil and permethrin (Effitix®, Virbac) in treating and preventing tick infestations of Ixodes ricinus in dogs. Methods: Twelve dogs were included in this randomized, controlled, blinded laboratory study. They were randomly allocated to two groups of six dogs each according to their pre-treatment live attached Ixodes ricinus tick count. On day 0,thedogsfromGroup2weretreatedwiththerecommendeddose of Effitix®, the dogs from Group 1 remained untreated. On days −2, 7, 14, 21, 28 and 35, all dogs were infested with 50 (±4) viable unfed adult Ixodes ricinus (20 ± 2 males, 30 ± 2 females). Ticks were removed and counted at 48 ± 2 hours post product administration or tick infestations. Results: Through the study, the tick attachment rates for the untreated group were greater than 25% demonstrating that adequate levels of infestation were reached on the control dogs. -

Draft Product Performance Test Guidelines OCSPP 810.3300
United States Office of Chemical Safety EPA 712-[insert number] Environmental Protection and Pollution Prevention [DRAFT DATE 4/4/19] Agency (7101) Draft Product Performance Test Guidelines OCSPP 810.3300: Treatments Topically Applied to Pets to Control Certain Invertebrate Ectoparasitic Pests NOTICE This guideline is one of a series of test guidelines established by the Office of Chemical Safety and Pollution Prevention (OCSPP) [formerly the Office of Prevention, Pesticides and Toxic Substances (OPPTS) prior to April 22, 2010], United States Environmental Protection Agency (US EPA) for use in testing pesticides and chemical substances to develop data for submission to the agency under the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601, et seq.), the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) (7 U.S.C. 136, et seq.), and section 408 of the Federal Food, Drug and Cosmetic (FFDCA) (21 U.S.C. 346a), referred to hereinafter as the harmonized test guidelines. The OCSPP test guidelines serve as a compendium of accepted scientific methodologies for research intended to provide data to inform regulatory decisions under TSCA, FIFRA, and/or FFDCA. This document provides guidance for conducting appropriate tests, and is also used by EPA, the public, and the companies that are required to submit data under FIFRA. These guidelines are not binding on either EPA or any outside parties, and the EPA may depart from them where circumstances warrant and without prior notice. The methods described in these guidelines are strongly recommended for generating the data that are the subject of the guidelines, but EPA recognizes that departures may sometimes be appropriate. -
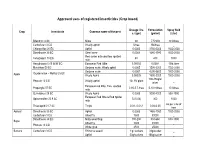
Approved Uses of Registered Insecticides (Crop Based)
Approved uses of registered insecticides (Crop based) Dosage / ha Formulation Spray fluid Crop Insecticide Common name of the pest a.i (gm) (gm/ml) (Liter) Bifenthrin 8 SC Mites 60 7.5ml/lit 10 lit/tree Carbofuran 3 CG Woolly aphid 5/tree 166/tree _ Chlorpyrifos 20 EC Aphid 0.0005 3750-5000 1500-2000 Dimethoate 30 EC Stem borer 0.0003 1485-1980 1500-2000 Red spider mite and two spotted Fenazaquin 10 EC 40 400 1000 mite Hexythiazox 5.45 W/W EC European Red Mite 0.00002 0.0004 10ltr./tree Malathion 50 EC Sanjose scale, Wooly aphid 0.0005 1500-2000 1500-2000 Sanjose scale 0.0007 4200-5600 1500-2000 Oxydemeton – Methyl 25 EC Apple Wooly Aphid 0.00025 1500-2000 1500-2000 100-150gm/ Phorate 10 CG Woolly aphid 10- 15/ plant _ plant European red Mite, Two spotted Propargite 57 EC 2.85-5.7 /tree 5-10 ml/tree 10 lit/tree mite Quinalphos 25 EC Wooly Aphid 0.0005 3000-4000 500-1000 European Red Mite & Red Spider Spiromesifen 22.9 SC 72(0.03) 300 1000 mite As per size of Thiacloprid 21.7 SC Thrips 0.01- 0.012 0.04-0.05 tree Apricot Dimethoate 30 EC Aphid 0.0003 1485-1980 1500-2000 Carbofuran 3 CG Shoot fly 1500 50000 _ Dimethoate 30 EC Milky weed bug 180-200 594-660 500-1000 Bajra Shoot fly 3000 30000 _ Phorate 10 CG White grub 2500 25000 _ Banana Carbofuran 3 CG Rhizome weevil 1 g/ suckers 33g/sucker _ Aphid 50g/suckers 166g/sucker _ Nematode 1.5g/suckers 50g/suckers _ Dimethoate 30 EC Aphid, Lace wing bug 0.0003 1485-1980 1500-2000 Tingyi bug 0.00025 1500-2000 1500-2000 Oxydemeton – Methyl 25 EC Aphids 0.0005 3000-4000 1500-2000 2.5 -1.25/ 25 -12.5/ Phorate 10 CG Aphid _ plant plant Quinalphos 25 EC Tingid bug 0.0005 3000-4000 500-1000 Phosalone 35 EC Aphid 500 1428 500-1000 Aphid 1000 33300 _ Barely Carbofuran 3 CG Jassid 1250 41600 _ Cyst nematode 1000 33300 _ Phorate 10 CG Aphid 1000 10000 _ Beans Chlorpyrifos 20 EC Pod borer , Black bug 600 3000 500-1000 Chlorantraniliprole 18.5 SC Pod borers 25 125 500 Chlorpyrifos 1.5 DP Helicoverpa armigera 375 25000 _ Azadirachtin 0.03 (300 PPM) Pod Borer _ _ _ Bacillus thuringiensis Var. -

Cyantraniliprole
PUBLIC RELEASE SUMMARY on the Evaluation of the New Active Constituent Cyantraniliprole in the Product DuPont Exirel Insecticide APVMA Product Number 64103 OCTOBER 2013 © Australian Pesticides and Veterinary Medicines Authority 2013 ISSN: 1443-1335 (electronic) ISBN: 978-1-922188-50-2 (electronic) Ownership of intellectual property rights in this publication Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Australian Pesticides and Veterinary Medicines Authority (APVMA). Creative Commons licence With the exception of the Coat of Arms and other elements specifically identified, this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence. This is a standard form agreement that allows you to copy, distribute, transmit and adapt this publication provided that you attribute the work. A summary of the licence terms is available from www.creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from www.creativecommons.org/licenses/by/3.0/au/legalcode. The APVMA’s preference is that you attribute this publication (and any approved material sourced from it) using the following wording: Source: licensed from the Australian Pesticides and Veterinary Medicines Authority (APVMA) under a Creative Commons Attribution 3.0 Australia Licence. In referencing this document the Australian Pesticides and Veterinary Medicines Authority should be cited as author, publisher and copyright owner. Use of the Coat of Arms The terms under which the Coat of Arms can be used are set out on the Department of the Prime Minister and Cabinet website (see www.dpmc.gov.au/guidelines). -

CYANTRANILIPROLE (263) the First Draft Was Prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand
Cyantraniliprole 361 CYANTRANILIPROLE (263) The first draft was prepared by Mr. David Lunn New Zealand Food Safety Authority, Wellington, New Zealand EXPLANATION Cyantraniliprole is a diamide insecticide with a mode of action (ryanodine receptor activation) similar to chlorantraniliprole and flubendiamide. It has root systemic activity with some translaminar movement and is effective against the larval stages of lepidopteran insects; and also on thrips, aphids, and some other chewing and sucking insects. Authorisations exist for the use of cyantraniliprole in Canada, Columbia, Malaysia, New Zealand, Vietnam and the CLISS countries in West Africa. Authorisations are also being progressed in Australia, Europe and USA under an OECD Joint Review exercise. Cyantraniliprole was scheduled by the Forty-fourth Session of the CCPR as a new compound for consideration by the 2013 JMPR. Residue and analytical aspects of cyantraniliprole were considered for the first time by the present meeting. The manufacturer submitted studies on metabolism, analytical methods, supervised field trials, processing, freezer storage stability, environmental fate in soil and rotational crop residues. In this evaluation, the values presented in the tables are as reported in the various studies, but in the accompanying text, they have generally been rounded to two significant digits. Abbreviations have also been used for the various cyantraniliprole metabolites mentioned in the study reports. These include: IN-F6L99 3-Bromo-N-methyl-1H-pyrazole-5-carboxamide IN-HGW87 -

Effects of Cyantraniliprole, a Novel Anthranilic Diamide Insecticide, Against Asian Citrus Psyllid Under Laboratory and Field Co
Submitted to: Correspondence to: Pest Management Science Lukasz L. Stelinski, Entomology and Nematology Department, Citrus Research & Education Center, University of Florida, 700 Experiment Station Road, Lake Alfred, FL 33850. Ph: 863 956 8851. Fax: 863 956 4631. Email: [email protected] Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions Siddharth Tiwari and Lukasz L. Stelinski* Entomology and Nematology Department, Citrus Research and Education Center, University of Florida, Lake Alfred, FL 33850 *Corresponding author Accepted Article This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/ps.3468. © 2012 Society of Chemical Industry Abstract BACKGROUND:The Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), is the most destructive pest of citrus in Florida. The development of insecticide resistance in several populations of D. citrihas been documented. There is an urgent need to develop and integrate novel tools for the successful management of D. citri and also to prevent the development of insecticide resistance. RESULTS:We investigated the effects of a relatively newer chemistry, cyantraniliprole, against D. citri. The contact toxicity of cyantraniliprole was 297 fold higher against D. citrithan its primary parasitoid,Tamarixia radiata(Hymenoptera: Eulophidae). D. citrisettled and fed less on cyantraniliprole-treated plants than controls at concentrations as low as 0.025 and 0.125 µg AI mL-1, respectively. D. citri egg production, first instar emergence and adult emergence were significantly reduced on plants treated with 0.25, 0.02 and 0.25 µg AI mL-1of cyantraniliprole, respectively, when compared with control plants. -

FY 2009-2012 Registration Review Schedule Summary
Registration Review: Summary of Planned Schedule for Opening Registration Review Dockets by Fiscal Year 2009 to 2012 December 15, 2008 EPA Office of Pesticide Programs: Registration Review Schedule Summary 12-15-08 Update Planned Schedule for Opening Registration Review Dockets by Fiscal Year (Oct. 1 - Sept. 30) FY 2009 Dockets FY 2010 Dockets FY 2011 Dockets FY 2012 Dockets (Actuals - Reviews Initiated) CONVENTIONAL PESTICIDES Acephate OP Aldicarb CA Ethalfluralin DN Benfluralin DN Azinphos-Methyl OP Aldoxycarb CA Oryzalin DN Butralin DN Chlorethoxyfos OP Carbaryl CA Prodiamine DN Pendimethalin DN Chlorpyrifos OP Carbofuran CA Fenvalerate PPS Trifluralin DN DDVP OP Formetanate HCl CA gamma Cyhalothrin PPS Acetamiprid NN DEF OP Methiocarb CA Imiprothrin PPS Clothianidin NN Dimethoate OP Methomyl CA lambda-Cyhalothrin PPS Dinotefuran NN Disulfoton OP Oxamyl CA Permethrin PPS Nitrapyrin NN Ethoprop OP Pirimicarb CA Piperonyl butoxide PPS Thiacloprid NN Fenitrothion OP Propoxur CA Tau-fluvalinate PPS Thiamethoxam NN Fosthiazate OP Thiodicarb CA Bensulfuron SU Cypermethrin PPS Malathion OP Copper Compounds: Group 2 CU Chlorimuron SU MGK-264 PPS Methamidophos OP Copper Salts CU Metsulfuron SU Prallethrin PPS Methidathion OP Copper Sulfate CU Sulfosulfuron SU Pyrethrin and derivatives PPS Methyl-Parathion OP 1RS, cis-Permethrin PPS Thifensulfuron SU Resmethrin PPS Mevinphos OP Allethrin stereoisomers PPS Tribenuron SU Sumethrin PPS Naled OP Bifenthrin PPS 2-Hydroxyethyl octyl sulfide Tefluthrin PPS Phorate OP Cyfluthrins PPS Ancymidol Tetramethrin -

Possible Enzymatic Mechanism Underlying Chemical Tolerance and Characteristics of Tolerant Population in Scapholeberis Kingi
Possible Enzymatic Mechanism Underlying Chemical Tolerance and Characteristics of Tolerant Population in Scapholeberis Kingi Makoto Ishimota ( [email protected] ) The Institute of Environmental Toxicology https://orcid.org/0000-0003-4686-0244 Mebuki Kodama The Institute of Environmental Toxicology Naruto Tomiyama The Institute of Environmental Toxicology Research Article Keywords: Cladocera, Insecticide, Tolerance, Acetylcholinesterase, Peroxidase, Superoxide dismutase, Multi-generational study, Field population. Posted Date: May 17th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-492302/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/24 Abstract To determine the potential effects of pesticides on aquatic organisms inhabiting a realistic environment, we explored the characteristics and mechanisms of chemical tolerance in Scapholeberis kingi. We established a chemical-tolerant population via continuous exposure to pirimicarb, an acetylcholinesterase (AChE) inhibitor, and examined the effects of pirimicarb concentration on the intrinsic growth rates (r) of tolerant cladocerans. We also explored the association between r and feeding rate and tested the involvement of antioxidant enzymes [peroxidase (PO) and superoxide dismutase] and AChE in pirimicarb sensitivity. S. kingi was continuously exposed to sublethal pirimicarb concentrations (0, 2.5, 5, and 10 µg/L) for 15 generations and changes (half maximal effective concentration at 48 h, 48 h-EC50) in chemical sensitivity were investigated. In the F14 generation, the sensitivity of the 10 µg/L group was three times lower than that of the control group, suggesting the acquisition of chemical tolerance. Moreover, r was signicantly and negatively correlated with 48 h-EC50, suggesting a tness cost for tolerance.