National Centre in Hiv Epidemiology & Clinical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Project Report
Identifying gaps in achieving the hiv elimination of HIV end among gay, bisexual, and other men who have sex with men in Australia project report Eithandee Aung1 Curtis Chan1 Skye McGregor1 Martin Holt2 Andrew E Grulich1 Benjamin R Bavinton1 1The Kirby Institute, UNSW Sydney 2The Centre for Social Research in Health, UNSW Sydney The Gaps Project Report 1 Identifying gaps in achieving the elimination of HIV transmission among gay, bisexual, and other men who have sex with men in Australia: The Gaps Project Report Authors: Eithandee Aung1 Curtis Chan1 Skye McGregor1 Martin Holt2 Andrew E Grulich1 Benjamin R Bavinton1 1The Kirby Institute, UNSW Sydney 2The Centre for Social Research in Health, UNSW Sydney © The Kirby Institute, UNSW Sydney, 2020 ISBN: 978-0-7334-3945-2 Suggested citation: Aung E, Chan C, McGregor S, Holt M, Grulich AE, Bavinton BR. (2020). Identifying gaps in achieving the elimination of HIV transmission among gay, bisexual, and other men who have sex with men in Australia: The Gaps Project Report. Sydney: Kirby Institute, UNSW Sydney. DOI: 10.26190/5f9f3f288a6ae. The Gaps Project Report 2 acknowledgements Contributors: • Rebecca Guy, Garrett Prestage, Praveena Gunaratnam, Jonathan King, Tobias Vickers, Prital Patel, Douglas Fraser, The Kirby Institute, UNSW Sydney • Limin Mao, The Centre for Social Research in Health, UNSW Sydney • Zhihong Gu, Ethnic Communities Council of Queensland • Brendan Kennedy, SA Health • Rick Varma, Sydney Sexual Health Centre • Jacqueline Kennedy, Alun Richards, Queensland Health • Darryl O’Donnell, -
Constitutional Recognition Relating to Aboriginal and Torres Strait Islander Peoples Submission 394 - Attachment 1
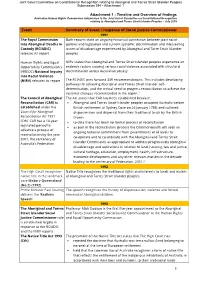
Joint Select Committee on Constitutional Recognition relating to Aboriginal and Torres Strait Islander Peoples Submission 394 - Attachment 1 Attachment 1 - Timeline and Overview of findings Australian Human Rights Commission Submission to the Joint Select Committee on Constitutional Recognition relating to Aboriginal and Torres Strait Islander Peoples – July 2018 Event Summary of event / response of Social Justice Commissioner 1991 The Royal Commission Both reports state an ongoing historical connection between past racist into Aboriginal Deaths in policies and legislation and current systemic discrimination and intersecting Custody (RCIADIC) issues of disadvantage experienced by Aboriginal and Torre Strait Islander releases its report peoples. Human Rights and Equal NIRV states that Aboriginal and Torres Strait Islander peoples experience an Opportunity Commission's endemic racism, causing serious racial violence associated with structural (HREOC) National Inquiry discrimination across Australian society. into Racist Violence (NIRV) releases its report The RCIADIC puts forward 339 recommendations. This includes developing pathways to achieving Aboriginal and Torres Strait Islander self- determination, and the critical need to progress reconciliation to achieve the systemic changes recommended in the report.1 The Council of Aboriginal The Act states that CAR has been established because: Reconciliation (CAR) is Aboriginal and Torres Strait Islander peoples occupied Australia before established under the British settlement at Sydney Cove -

Plasma HIV-1 Tropism and the Risk of Short-Term Clinical Progression to AIDS Or Death
Plasma HIV-1 Tropism and the Risk of Short-Term Clinical Progression to AIDS or Death Casadellà, Maria; Cozzi-Lepri, Alessandro; Phillips, Andrew; Noguera-Julian, Marc; Bickel, Markus; Sedlacek, Dalibor; Zilmer, Kai; Clotet, Bonaventura; Lundgren, Jens D; Paredes, Roger; EuroSIDA in EuroCoord Published in: PloS one DOI: 10.1371/journal.pone.0166613 Publication date: 2017 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Casadellà, M., Cozzi-Lepri, A., Phillips, A., Noguera-Julian, M., Bickel, M., Sedlacek, D., Zilmer, K., Clotet, B., Lundgren, J. D., Paredes, R., & EuroSIDA in EuroCoord (2017). Plasma HIV-1 Tropism and the Risk of Short- Term Clinical Progression to AIDS or Death. PloS one, 12(1), [e0166613]. https://doi.org/10.1371/journal.pone.0166613 Download date: 28. Sep. 2021 RESEARCH ARTICLE Plasma HIV-1 Tropism and the Risk of Short- Term Clinical Progression to AIDS or Death Maria Casadellà1,2*, Alessandro Cozzi-Lepri3, Andrew Phillips3, Marc Noguera-Julian1,2,4, Markus Bickel5, Dalibor Sedlacek6, Kai Zilmer7, Bonaventura Clotet1,2,4,8, Jens D. Lundgren9, Roger Paredes1,2,4,8, EuroSIDA in EuroCOORD¶ 1 IrsiCaixa AIDS Research Institute, Badalona, Catalonia, Spain, 2 Universitat Autònoma de Barcelona, Catalonia, Spain, 3 Royal Free Hospital, London, United Kingdom, 4 Universitat de Vic-Universitat Central de Catalunya, Vic, Catalonia, Spain, 5 Goethe University, Frankfurt/Main, Germany, 6 Charles University a1111111111 Hospital, Plzen, Česka Republika, 7 West-Tallinn Central Hospital, Tallinn, Estonia, 8 HIV Unit, Hospital a1111111111 Universitari Germans Trias i Pujol, Badalona, Catalonia, Spain, 9 CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark a1111111111 a1111111111 ¶ Membership of this author group is listed in the Acknowledgments. -

Winnunga Newsletter June 2017
Aboriginal Health in Aboriginal Hands Winnunga News ISSN 2206-3080 J U N E 2 0 1 7 Inside this Issue: Introduction to Winnunga “Winnunga 101” 2, 3, 4 What a Surprise—Aboriginal CEO Update Person First to be Locked Up Under New Bail Laws 5 This year’s Sorry Day Bridge Walk was held on Friday 26 May. The Steven Freeman death esponse event was dedicated to Steven Freeman, a young Aboriginal man chair Russell Taylor not afraid to 'shake up' officials 6,7 who sadly passed away whilst in custody at the AMC, on the same day as our Sorry Day Bridge Walk last year. I was particularly Clinical Services Updates 8, 9 humbled to have Steven’s mother Narelle and her family present Sorry Day Bridge Walk 9, 10, 11 with us on the day. Julie Tongs OAM, CEO It’s a Slam Dunk for the Winnunga Warriors Sports The reasons for the Winnunga Sorry Day Bridge Walk, are now Club 12, 13, 14 more relevant and important than ever. Here in Canberra 25% of Living National Treasure Dr all children in Out of Home care between the ages of 0 to 17, are Naomi Mayers OAM 15 Aboriginal and/or Torres Strait Islanders. An Aboriginal child born World No Tobacco Day in the ACT is 20 times more likely to be taken into care than a non- Winnunga Says No To Smoking Aboriginal child, yet we only make up around 2% of the population. 16, 17, 18, 19, 20 No More Boondah Program Here in Canberra we also have the highest rate of Aboriginal people incarcerated nationally. -

Correlating HIV Tropism with Immunological Response Under Cart
!"#$%&'%()*+,& -$%%,+"()./& 012& 3%$4)56& 7)(8& 1669.$+$/)*"+& :,54$.5,& ;.<,%& *':3& -$%,*,4($%&;5,&".<&=)5,"5,&>%$/%,55)$.& & !"#$$%& '()%*+,& -*(./012(& 3456.07899"$:%*;,& !<*=& '6.0>,& ?%*0& @"*=0%A1207B*01"5"C,& 3(D0.%& E%*$FG,& @$()F1& ?(*:0.%::0H,& ?(.I%$& '(::%=(FJ,& K5"L(1& M$0L2(0:+,& (.)& :5%& 3N011&BOP&Q"5"*:&3:I)F& !"#$%&'$()* +,)#$#-./* 0%1()23%42* 5,#3%6,&,4%* 7* 8%2%)91$(2:/* ;4,<%)9,2.* #=* 5(9%$/* >??@* 5(9%$/* AB,2:%)$(46C* DAB,99* EF+* G#H#)2* A2'6.* IAEGAJ* 0(2(* G%42%)/* ;4,<%)9,2.* E#91,2($* K('9(44%/* !?!L* K('9(44%/* AB,2:%)$(46* MF492,2'2%* #=* "%6,&($* +,)#$#-./* N(2,#4($* G%42%)* =#)* O%2)#<,)'9%9/* ;4,<%)9,2.* #=* PQ),&H/* R?LS* PQ),&H/* AB,2:%)$(46* >F492,2'2%*=#)*F4=%&2,#'9*0,9%(9%9/*;4,<%)9,2.*#=*5%)4%/*M?!?*5%)4/*AB,2:%)$(46* L;4,<%)9,2.*E#91,2($9*IE;TJ/*K(U#)(2#).*#=*+,)#$#-./*!D?L*T%4V<%/*AB,2:%)$(46W* X0%1()23%42*#=*0%)3(2#$#-./*O%-,#4($*E#91,2($*#=*5%$$,4:#4(/*XL?!*5%$$,4:#4(/*T%4%<(/*AB,2:%)$(46W* S0,<,9,#4* #=* F4=%&2,#'9* 0,9%(9%9* (46* E#91,2($* Y1,6%3,#$#-./* ;4,<%)9,2.* E#91,2($* 5(9%$/* >?M!* 5(9%$/* AB,2:%)$(46* * 8D1:*(4:&N"*)&4"I.:R&+ST& K%U:&N"*)&4"I.:R&>V>+& & Q"**%1W".)%.4%R&& X"%$$%YD()%*ZI.0D(1Y45,&VVC+&H;&;HJ&VS&T+& & ! "! '?5(%"*(& '(42=*"I.)R&[(:0%.:1&0.9%4:%)&N0:5&:5%&5IL(.&0LLI.")%9040%.4F&A0*I1&:FW%&+&\BOP7+]& L(F& %UW%*0%.4%& ".$F& 1ID"W:0L($& Q^C& 4%$$& *%4"A%*F& N50$%& :*%(:%)& N0:5& 4"LD0.(:0".& :5%*(WF& \48_K]Y& `0::$%& 01& 2."N.& (D"I:& N5%:5%*& A0*($& W*"W%*:0%1& 1I45& (1& 4%$$& :*"W01L& 4"I$)& W$(F& (& *"$%& 9"*& 1I45& 0.4"LW$%:%& 0LLI.%& *%1W".1%Y& K5I1& :501& 1:I)F& N(1& -

Coreceptor Tropism Assays
CORECEPTOR TROPISM ASSAYS Trofile® and Trofile® DNA provide critical information for informed treatment decisions HIV-1 can attach to human cells either by using the CCR5 coreceptor, the CXCR4 coreceptor, or both (dual/mixed). Tropism testing determines how the virus can attach to the cells in a given patient. Possible tropism results are R5, DM, X4, and X4 near the limit of detection (NLOD). Why does my patient’s tropism matter? Trofile® DNA HIV tropism results can help you develop a personalized Applies the proven performance of Trofile to cell-associated treatment plan for your patient. Appropriate use of CCR5 viral DNA antagonists including maraviroc requires that an HIV tropism Consider Trofile® DNA when a patient’s viral load is test be performed before initiation of therapy.1 undetectable, tropism is unknown, and substitution with a CCR5 antagonist-containing regimen is desired. Regimen substitution may be considered when2 Trofile® • Laboratory results or clinical adverse events necessitate A highly sensitive assay that provides critical information when a change7,8 selecting a treatment regimen containing maraviroc • Patient exhibits intolerance to the current regimen7 • Trofile was utilized to identify treatment candidates in the • There is concern regarding the long-term effects of the maraviroc multicenter clinical trials.1 current regimen8 • Both the current DHHS and IDSA guidelines recommend tropism testing before initiation of treatment with a CCR5 antagonist.2,3 GenoSure Archive® Plus Trofile® DNA • Trofile is the only commercially available tropism assay Comprehensive suppression management profile that has been clinically validated through use in Phase 2 Designed to provide a comprehensive assessment of and Phase 3 clinical studies to identify CCR5 five antiretroviral drug classes (GenoSure Archive: NRTIs, antagonist candidates.4-6 NNRTIs, PIs, INIs and Trofile DNA: CCR5 antagonist) to facilitate regimen simplification or switches in the setting of Virologic suppression. -

COVID-19 Series: Report 1 COVID-19 How Australia’S Health and Medical Research Sector Is Responding 2
COVID-19 Series: Report 1 COVID-19 How Australia’s health and medical research sector is responding 2 Research Australia is the national alliance representing the entire How Australia’s health and medical research sector is responding How Australia’s health and medical research COVID-19: pipeline from the laboratory through to the patient and the marketplace. Table of Contents 3 Foreword 8 New South Wales 18 UNDERSTANDING THE CORONAVIRUS SARS-COV-2 21 Introduction 9 First release of the gene sequence of COVID-19 21 Detecting genetic variation 21 COVID-19 surveillance 21 What we have learned 10 Co-infection information for flu season 21 COVID-19 sub-strains and transmission rates 21 Clinician decision-making tool Health and medical research to adapt to changing conditions 22 and innovation in Australia 12 Evidence base to address challenges in the medical system from COVID-19 22 Enabling medical research be Australian Capital Territory 14 undertaken 30-80 times faster 22 UNDERSTANDING THE CORONAVIRUS THERAPIES 22 SARS-COV-2 15 Nanotechnology to deliver COVID Mapping the spread of COVID-19 in real time 15 treatment via nose and mouth 22 THERAPIES 15 Engineering COVID-19 antibodies 22 How Australia’s health and medical research sector is responding How Australia’s Factors for survival of patients Delivering antivirals to the lungs for treatment 23 with Acute Respiratory Distress Syndrome 15 Testing repurposing existing drugs 23 Examining chronic disease impact 23 TESTING AND DIAGNOSTICS 15 Monitoring sewage as an early warning system 15 -

Supporting Collaborative Research Infrastructure Or Network Lab Project
Office of the Pro-Vice-Chancellor (Research Infrastructure) Research Infrastructure Scheme: Supporting Collaborative Research Infrastructure or Network Lab Project Application for Funding in 2018 Faculty Medicine Family Name, Lead Investigator Kelleher, Anthony (Please only name ONE person here) ☒ Faculty Infrastructure Project ☐ Mark Wainwright Analytical Centre Infrastructure Project ☐ Network Lab Project Type For any of the above, please indicate: ☒ 1-year or ☐ 2-year project Project title (Please chose a title descriptive of 4 laser, 16-colour cell sorter and class II biosafety cabinet the infrastructure requested) Amount requested centrally (ex GST) 2018 $495 000 2019 $ (For 2-year projects, detail the amount requested in each year) School / Faculty approved contribution, if 2018 $ 2019 $ applicable 1 RESEARCH INFRASTRUCTURE SCHEME: Supporting Collaborative Research Application Form for Funding Support in 2018 When completing this application, please refer to the scheme’s guidelines. Applications must be lodged with the relevant School Office as a single pdf file by 18 September 2017 and must include: • A completed and signed Site and Services Checklist: The lead investigator is asked to complete the checklist, sign the document as ‘Project Lead’ and obtain sign-off by the relevant Head of School as ‘Sponsor’ (refer to the checklist for instructions). • Suppliers’ quotes for items to be purchased: See Section 8 for details. The Site and Services Checklist, quote templates, guidelines and other resources for this scheme are available at https://research.unsw.edu.au/unsw-research-infrastructure-scheme. Additional contacts for advice and assistance are listed at the end of this form. Use this form for Faculty or MWAC Infrastructure and Network Lab Projects only. -

Determination of HIV-1 Coreceptor Tropism Using Proviral DNA In
Baumann et al. AIDS Research and Therapy (2015) 12:11 DOI 10.1186/s12981-015-0055-x RESEARCH Open Access Determination of HIV-1 coreceptor tropism using proviral DNA in women before and after viral suppression Russell E Baumann1, Amy A Rogers1, Hasnah B Hamdan1, Harold Burger2, Barbara Weiser2, Wei Gao3, Kathryn Anastos3, Mary Young4, William A Meyer III5, Rick L Pesano1 and Ron M Kagan1* Abstract Background: An HIV-1 tropism test is recommended prior to CCR5 antagonist administration to exclude patients harboring non-R5 virus from treatment with this class of antiretrovirals. HIV-1 tropism determination based on proviral DNA (pvDNA) may be useful in individuals with plasma viral RNA suppression. We developed a genotypic tropism assay for pvDNA and assessed its performance in a retrospective analysis of samples collected longitudinally. Results: We randomly selected paired plasma/PBMC samples from the Women’s Interagency HIV Study with plasma viral load ≥5,000 cp/mL at time 1 (T1), undetectable viral load maintained for ≥1 year and CD4+ >200 cells/μLattime 2 (T2). pvDNA was isolated from cryopreserved PBMCs. Sequences were analyzed in triplicate from 49/50 women, with tropism assigned using the geno2pheno (g2p) algorithm. The median time between T1 and T2 was 4.1 years. CXCR4-using virus was detected in 24% of the RNA samples and 33% of the pvDNA samples at T1, compared to 37% of the pvDNA samples at T2. Concordance between plasma RNA and pvDNA tropism was 88% at T1 and 80% at T2. The g2p scores for RNA (T1) vs DNA (T1, T2) were strongly correlated (Spearman rho: 0.85 (T1); 0.78 (T2)). -

Accepted Manuscript
Progress towards elimination of hepatitis C infection among people who inject drugs in Australia: The ETHOS Engage Study Heather Valerio,1 Maryam Alavi,1 David Silk,1 Carla Treloar,2 Marianne Martinello,1 Andrew Milat,3,4 Adrian Dunlop,5,6 Jo Holden,7 Charles Henderson,8 Janaki Amin, 1,9 Phillip Read, 1,10 Philippa Marks, 1 Louisa Degenhardt, 11 Jeremy Hayllar,12 David Reid,13 Carla Gorton,14 Thao Lam,15 Gregory J Dore1, and Jason Grebely1 on behalf of the ETHOS II Study Group 1 The Kirby Institute, UNSW Sydney, Sydney, NSW, Australia Downloaded from https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciaa571/5838577 by Jules Levin on 19 May 2020 2 Centre for Social Research in Health, UNSW Sydney, Sydney, NSW, Australia 3 Centre for Epidemiology and Evidence, NSW Health, NSW, Australia 4 School of Public Health, University of Sydney, NSW, Australia 5 Centre for Translational Neuroscience and Mental Health, Hunter Medical Research Institute & University of Newcastle, Newcastle, NSW, Australia 6 Drug and Alcohol Clinical Services, Hunter New England Local Health District, Newcastle, NSW, Australia 7 Population Health Strategy & Performance, NSW Health, NSW, Australia 8 NSW Users and AIDS Association, NSW, Australia 9 Macquarie University, Sydney, NSW, Australia 10 Kirketon Road Centre, Sydney, NSW, Australia 11 National Drug and Alcohol Research Centre, UNSW Sydney, Sydney, NSW, Australia 12 Alcohol and Drug Service, Metro North Mental Health, Metro North Hospital and Health Service, Brisbane, QLD, Australia 13 The Orana Centre, Illawarra Shoalhaven LHD, Wollongong, NSW, Australia 14 Cairns Sexual Health Service, Cairns, QLD, Australia 15 Drug Health, Western Sydney Local Health District, Sydney, NSW, Australia Corresponding author: Heather Valerio The Kirby Institute, UNSW Sydney, Sydney, New South Wales, Australia Wallace Wurth Building, High Street, Kensington, NSW, 2052, Australia. -

CCHCS Care Guide: Human Immunodeficiency Virus (HIV)
September 2021 CCHCS Care Guide: Human Immunodeficiency Virus (HIV) SUMMARY DECISION SUPPORT PATIENT EDUCATION/SELF MANAGEMENT ALL HIV INFECTED PATIENTS MUST BE MANAGED BY A CCHCS HIV SPECIALIST GOALS • Offer HIV screening to all • Ensure a sexual history and appropriate risk reduction counseling is performed • Refer all patients with HIV to HIV specialists as by a primary care team member for every patient with HIV at least annually. soon as possible • Initiate antiretroviral therapy (ART) for all patients with HIV as soon as possible • Identify newly diagnosed cases of HIV/Acquired • Screen and evaluate the patients with substance use disorder as a Immunodeficiency Syndrome (AIDS) transmission risk factor (see CCHCS Substance Use Disorder Care Guide) • Identify acute HIV seroconversion ALERTS Inappropriate or suboptimal treatment regimens Red Flags • Patients receiving only one HIV medication rather than a multi-drug ANY CD4 CD4 <200 CD4 <100 combination (note that some co-formulations exist) • New onset fevers • Dyspnea • Headache • Patients on treatment for months with a persistently detectable viral • Weight loss >10% • Cough • Blurry or lost load • Fatigue • Fevers vision • Patients with CD4 <200 cells/mm3 who are not on Pneumocystis • Skin lesions • Diarrhea jiroveci (PCP) prophylaxis (see page 6) • Night sweats DIAGNOSTIC CRITERIA/EVALUATION (SEE PAGE 2 FOR HIV TESTING ALGORITHM) D Consider HIV in the following circumstances: • Patients with known high risk behaviors prior to or during incarceration (tattoos, injection drug use, -

The Medical Associations Couple Husband & Wife Presidents, CSANZ’S Prof
THE OFFICIAL JOURNAL OF ASSOCIATIONS FORUM EDITION 45 APRIL 2016 ASSOCIATIONS INFORMING ASSOCIATIONS AND CHARITIES IN AUSTRALIA, NEW ZEALAND AND ASIA The Medical Associations Couple Husband & wife Presidents, CSANZ’s Prof. Andrew MacIsaac & ASA’s Dr Maree Barnes ASSOCIATION CHANGES Australian Dental Prosthetists Association Australasian College of Physical Scientists & Engineers in Medicine Wound Management Association Australia Australian Resident Accommodation Managers Association Australian Airports Association Boating Industry Association Institute of Professional Editors ◊ Spotlight on AF services and new membership offerings ◊ ACT elegibility for charitable status tightened ◊ AF’s Keynote Lunch with the NSW Treasurer ISSN 1838-8507 AssociationsEdition45 DRAFT 3.indd 1 14/03/2016 3:08 PM AssociationsEdition45 DRAFT 3.indd 2 14/03/2016 3:08 PM In this Issue... NEWS SALARY SURVEY Board News Associations Forum 4 ASSOCIATIONS is published by Salary Survey 2016 19 Association News 6 Associations Forum Pty Ltd PO Box 810, Artarmon NSW 1570 Australia P: +61 2 9904 8200 F: +61 2 9411 8585 COVER STORY SERVICES www.associations.net.au A review of Associations Forum http//twitter.com/assocforumau Married Presidents of ASA services and success stories 20 Publisher: John Peacock and Cardiac Society 10 Editor: Philippa Shelley Jones [email protected] ASSOCIATION UPDATE APPOINTMENTS Design: thedesigngroup + DEPARTURES www.thedesigngroup.com.au Recent developments including Printing & Distribution: OPUS Group ACT charity definition, Including