CMS Snapshot November 19-December 3, 2020 Delivered to You by the Partner Relations Group in the Office of Communications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
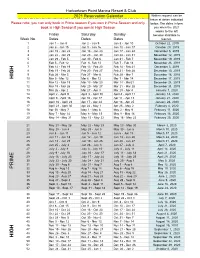
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

Peoria Unified School District 6-Day Rotation Schedule
Peoria Unified School District 2020 – 2021 School Year Six-Day Rotation Schedule August 2020 January 2021 Day 1: August 17, 19 AM, 26 PM and 27 Day 1: January 6 PM, 11 and 22 Day 2: August 18 and 28 Day 2: January 12, 13 AM, 20 PM and 25 Day 3: August 23 and 31 Day 3: January 4, 14, 26 and 27 AM Day 4: August 21 Day 4: January 5, 15 and 28 Day 5: August 24 Day 5: January 7, 19 and 29 Day 6: August 25 Day 6: January 8 and 21 September 2020 February 2021 Day 1: September 8, 18 and 29 Day 1: February 2, 12 and 25 Day 2: September 2 AM, 9 PM, and 21 Day 2: February 4, 16 and 26 Day 3: September 11, 16 AM and 23 PM Day 3: February 3 PM, 5 and 18 Day 4: September 1, 14, 24 and 30 AM Day 4: February 8, 10 AM, 17 PM and 19 Day 5: September 3, 15 and 25 Day 5: February 9, 22 and 24 AM Day 6: September 4, 17 and 28 Day 6: February 1, 11 and 23 October 2020 March 2021 Day 1: October 9 and 22 Day 1: March 8, 25 and 31 AM Day 2: October 1, 13 and 23 Day 2: March 9 and 26 Day 3: October 2, 15 and 26 Day 3: March 1, 11 and 29 Day 4: October 5, 7 PM, 16 and 27 Day 4: March 2, 12 and 30 Day 5: October 6, 14 AM, 21 PM and 29 Day 5: March 3 PM, 4 and 22 Day 6: October 8, 20, 28 AM and 30 Day 6: March 5, 10 AM, 23 and 24 PM November 2020 April 2021 Day 1: November 2, 12 and 20 Day 1: April 5, 7 PM, 15 and 27 Day 2: November 3, 13 and 30 Day 2: April 6, 14 AM, 16, 21 PM and 29 Day 3: November 5 and 16 Day 3: April 8, 19, 28 AM and 30 Day 4: November 6 and 17 Day 4: April 9 and 20 Day 5: November 9 and 18 Day 5: April 1, 12 and 22 Day 6: November 4 PM, 10 and 19 Day 6: April 2, 13 and 26 December 2020 May 2021 Day 1: December 7, 15 and 16 AM Day 1: May 7 and 18 Day 2: December 8 and 17 Day 2: May 10 and 19 Day 3: December 1 and 9 Day 3: May 5 PM, 11 and 20 Day 4: December 2 and 10 Day 4: May 3, 12 AM, 13 Day 5: December 3 and 11 Day 5: May 4 and 14 Day 6: December 4 and 14 Day 6: May 6 and 17 EARLY RELEASE or MODIFIED WEDNESDAY • Elementary schools starting at 8 a.m. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
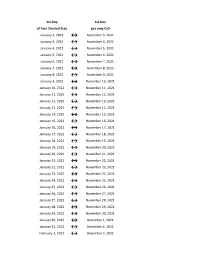
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 2, 2022 ↔ November 3, 2021 January 3, 2022 ↔ November 4, 2021 January 4, 2022 ↔ November 5, 2021 January 5, 2022 ↔ November 6, 2021 January 6, 2022 ↔ November 7, 2021 January 7, 2022 ↔ November 8, 2021 January 8, 2022 ↔ November 9, 2021 January 9, 2022 ↔ November 10, 2021 January 10, 2022 ↔ November 11, 2021 January 11, 2022 ↔ November 12, 2021 January 12, 2022 ↔ November 13, 2021 January 13, 2022 ↔ November 14, 2021 January 14, 2022 ↔ November 15, 2021 January 15, 2022 ↔ November 16, 2021 January 16, 2022 ↔ November 17, 2021 January 17, 2022 ↔ November 18, 2021 January 18, 2022 ↔ November 19, 2021 January 19, 2022 ↔ November 20, 2021 January 20, 2022 ↔ November 21, 2021 January 21, 2022 ↔ November 22, 2021 January 22, 2022 ↔ November 23, 2021 January 23, 2022 ↔ November 24, 2021 January 24, 2022 ↔ November 25, 2021 January 25, 2022 ↔ November 26, 2021 January 26, 2022 ↔ November 27, 2021 January 27, 2022 ↔ November 28, 2021 January 28, 2022 ↔ November 29, 2021 January 29, 2022 ↔ November 30, 2021 January 30, 2022 ↔ December 1, 2021 January 31, 2022 ↔ December 2, 2021 February 1, 2022 ↔ December 3, 2021 1st Day 1st Day of Your Desired Stay you may Call February 2, 2022 ↔ December 4, 2021 February 3, 2022 ↔ December 5, 2021 February 4, 2022 ↔ December 6, 2021 February 5, 2022 ↔ December 7, 2021 February 6, 2022 ↔ December 8, 2021 February 7, 2022 ↔ December 9, 2021 February 8, 2022 ↔ December 10, 2021 February 9, 2022 ↔ December 11, 2021 February 10, 2022 ↔ December 12, 2021 February -

Federal Register/Vol. 85, No. 224/Thursday, November 19, 2020
74196 Federal Register / Vol. 85, No. 224 / Thursday, November 19, 2020 / Proposed Rules DEPARTMENT OF HOMELAND considered by DHS and USCIS. Note: Implementing CAT and Deferral of SECURITY Comments received after December 21, Removal Under Regulations 2020 on the proposed rulemaking rather Implementing CAT 8 CFR Parts 106, 241 and 274a than those specific to the collection of E. Employment Authorization F. Biometric Submission [CIS No. 2653–19; DHS Docket No. USCIS– information will not be considered by V. Discussion of the Proposed Rule 2019–0024] DHS and USCIS. A. Eligibility for Employment ADDRESSES: You may submit comments Authorization for Aliens on Orders of RIN 1615–AC40 on the entirety of this proposed Supervision rulemaking package, identified by DHS B. USCIS Evidentiary Requirements Employment Authorization for Certain C. Biometric Submission and Criminal Classes of Aliens With Final Orders of Docket No. USCIS–2019–0024, through the Federal eRulemaking Portal: http:// History Removal D. Aliens Granted Deferral of Removal www.regulations.gov. Follow the Under the Regulations Implementing AGENCY: Department of Homeland website instructions for submitting CAT Security. comments. E. Effective Date of the Final Rule ACTION: Notice of proposed rulemaking. Comments submitted in a manner F. Additional Amendments other than the one listed above, VI. Statutory and Regulatory Requirements SUMMARY: The U.S. Department of including emails or letters sent to DHS A. Executive Orders 12866 (Regulatory Homeland Security (DHS) is proposing or USCIS officials, will not be Planning and Review) and 13563 to eliminate employment authorization considered comments on the proposed (Improving Regulation and Regulatory eligibility for aliens who have final rule and may not receive a response Review) 1. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
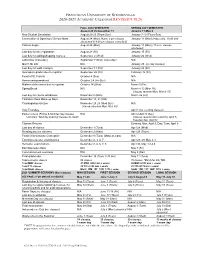
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

November 19, 2020
Thursday, November 19, 2020 Mayor Domenic J. Sarno, in the best interests of our residents and business community, continues his daily morning meetings with his cabinet heads as they work 24/7 to facilitate and coordinate with all of our local, state, federal, and public and private partners in response to the COVID-19 situation. Below are the most recent updates as reported from each City Departments. Mayor’s Office Continues to coordinate the city’s efforts with all stakeholders (state, federal, hospital and medical facilities, public and private partners, etc.) in response of services and relief and recovery measures Continuing to monitor the COVID-19 situation closely so that as the city continues with phased reopening aspects Continues to stress the need for additional target testing in order to build strong public, business, consumer, and hospital confidences to identify, isolate, contact trace, mitigate and heal potential hot spot areas City Hall and all municipal buildings have reopened, by appointment only and with additional restrictions, as part of the city’s Phased reopening plans . Temperature checks and face coverings needed to enter city buildings to proactively protect and preserve our workforce . Continuing to encourage residents and businesses to conduct city related business by phone or through the city’s website Continues to coordinate with cabinet and department heads on reopening aspects of all municipal buildings Heartfelt thank you to our Springfield Community for stepping up with PPE donations, allowing the city to outfit our dedicated police, fire and frontline workers with the appropriate safety equipment Governor Baker announced new initiatives to mitigate the spread of COVID-19 o Issued Travel Order for all travelers entering Massachusetts that took effect on August 1st o Current list of lower-risk states include: . -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

2021 Subdivision Calendar
Planning Department Development Review – Major Subdivision Applications First Plat Review 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Deadline for Staff Planning Board BOC Meeting Planning Department Review Deadline Postponement Meeting Date* Date** December 18, 2020 December 31 January 13 January 22 February 2 March 15 January 15 January 29 February 10 February 19 March 2 April 19 February 19 March 5 March 17 March 26 April 6 May 17 March 19 April 2 April 14 April 23 May 4 June 21 April 16 April 30 May 12 May 21 June 1 July 19 May 21 June 4 June 16 June 25 July 6 August 16 June 18 July 2 July 14 July 23 August 3 September 20 July 23 August 6 August 18 August 27 September 7 October 18 August 20 September 3 September 15 September 24 October 5 November 15 September 17 October 1 October 13 October 22 November 2 December 20 October 22 November 5 November 17 November 26 December 7 ▪ January 18, 2022 November 19 December 3 December 15 ▪ December 22 ▪ January 4, ▪ February 21, 2022 2022 ▪ ▪ ▪ December 17 December 31, January 12, ▪ January 21, ▪ February 1, March 21, 2021 2022 2022 2022 2022 * Planning Board Has Two (2) Meetings to Make Recommendation ** Board of Commissioners Has Up to Four (4) Meetings to Make Decision ▪ 2021 Dates are Tentative – Meeting Dates Will Be Set in December 2021 With Adoption of 2022 Meeting Calendars Construction/Final Plat Review – Administrative* 2021 Deadlines Submittal to Staff Sufficiency TRC Meeting Date Planning Review Deadline Department For TRC December 23, 2020 January 6 January 13 January -

Meeting Notice
SECURITIES AND EXCHANGE COMMISSION [Release Nos. 33-10885; 34-90338; File No. 265-28] Investor Advisory Committee Meeting AGENCY: Securities and Exchange Commission. ACTION: Notice of public meeting. SUMMARY: The Securities and Exchange Commission Investor Advisory Committee, established pursuant to Section 911 of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, is providing notice that it will hold a public meeting. The public is invited to submit written statements to the Committee. DATES: The meeting will be held on Thursday, December 3, 2020 from 10:00 a.m. until 4:00 p.m. (ET). Written statements should be received on or before December 3, 2020. ADDRESSES: The meeting will be conducted by remote means and/or at the Commission’s headquarters, 100 F St NE, Washington, DC 20549. The meeting will be webcast on the Commission’s web site at www.sec.gov. Written statements may be submitted by any of the following methods: Electronic Statements . Use the Commission’s Internet submission form (http://www.sec.gov/rules/other.shtml); or . Send an email message to [email protected]. Please include File No. 265-28 on the subject line; or 1 Paper Statements . Send paper statements to Vanessa A. Countryman, Secretary, Securities and Exchange Commission, 100 F Street, NE, Washington, DC 20549-1090. All submissions should refer to File No. 265-28. This file number should be included on the subject line if email is used. To help us process and review your statement more efficiently, please use only one method. Statements also will be available for website viewing and printing in the Commission’s Public Reference Room, 100 F Street, NE, Room 1503, Washington, DC 20549, on official business days between the hours of 10:00 a.m. -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January