Progesterone by the Guinea-Pig Placenta in Vitro
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pregnenolone Introduced 1997
Product Information Sheet – September 2016 Pregnenolone Introduced 1997 What Is It? • This product should not be taken by individuals with healthy levels of pregnenolone. Pregnenolone, 3-alpha-hydroxy-5-beta-pregnen-20-one, is a precursor to over 150 steroid hormones and is produced naturally in • Pregnenolone is best utilized by individuals over 40, and should the body from cholesterol. not be used to enhance athletic ability or endurance. Uses For Pregnenolone Are There Any Precautions Or Potential Side Memory Support: Animal studies have reported that pregnenolone Effects? may help to enhance memory by modulating N-methyl-D-aspartate Precautions: (NMDA) and gamma aminobutyrate (GABA) receptor activity in the brain. One study suggested that pregnenolone helped promote • NOT FOR USE BY INDIVIDUALS UNDER THE AGE OF 18. post-training task learning and memory.* • DO NOT USE IF PREGNANT OR NURSING. Immune Health: One study indicated that the 7-hydroxy metabolites • KEEP OUT OF REACH OF CHILDREN. from pregnenolone may help promote healthy immune system • Consult a physician or licensed qualified health professional before response.* using this product if you have, or have a family history of breast Mood Support: Pregnenolone has been reported to help promote cancer, prostate cancer, prostate enlargement, heart disease, low feelings of emotional well-being. One study suggested that “good” cholesterol (HDL), or if you are using any other dietary pregnenolone supported positive mood and feelings of motivation supplement, prescription drug, or over-the-counter drug. by mediating dopamine release.* • Do not exceed the recommended serving. Exceeding the What Is The Source? recommended serving may cause serious adverse health effects. -

The Organic Chemistry of Drug Synthesis
The Organic Chemistry of Drug Synthesis VOLUME 2 DANIEL LEDNICER Mead Johnson and Company Evansville, Indiana LESTER A. MITSCHER The University of Kansas School of Pharmacy Department of Medicinal Chemistry Lawrence, Kansas A WILEY-INTERSCIENCE PUBLICATION JOHN WILEY AND SONS, New York • Chichester • Brisbane • Toronto Copyright © 1980 by John Wiley & Sons, Inc. All rights reserved. Published simultaneously in Canada. Reproduction or translation of any part of this work beyond that permitted by Sections 107 or 108 of the 1976 United States Copyright Act without the permission of the copyright owner is unlawful. Requests for permission or further information should be addressed to the Permissions Department, John Wiley & Sons, Inc. Library of Congress Cataloging in Publication Data: Lednicer, Daniel, 1929- The organic chemistry of drug synthesis. "A Wiley-lnterscience publication." 1. Chemistry, Medical and pharmaceutical. 2. Drugs. 3. Chemistry, Organic. I. Mitscher, Lester A., joint author. II. Title. RS421 .L423 615M 91 76-28387 ISBN 0-471-04392-3 Printed in the United States of America 10 987654321 It is our pleasure again to dedicate a book to our helpmeets: Beryle and Betty. "Has it ever occurred to you that medicinal chemists are just like compulsive gamblers: the next compound will be the real winner." R. L. Clark at the 16th National Medicinal Chemistry Symposium, June, 1978. vii Preface The reception accorded "Organic Chemistry of Drug Synthesis11 seems to us to indicate widespread interest in the organic chemistry involved in the search for new pharmaceutical agents. We are only too aware of the fact that the book deals with a limited segment of the field; the earlier volume cannot be considered either comprehensive or completely up to date. -

| Secretion !------Cortisol Cortisol US 7,053,228 B2 Page 2
US007053228B2 (12) United States Patent (10) Patent No.: US 7,053,228 B2 Burton et al. (45) Date of Patent: May 30, 2006 (54) SULFUR ANALOGUES OF (52) U.S. Cl. ...................... 552/512; 514/179; 514/180; 21-HYDROXY-6,19-OXIDOPROGESTERONE 552/510; 549/29: 549/41 (21OH-60P). FOR TREATING EXCESS OF (58) Field of Classification Search ................ 514/179, GLUCOCORTICODS 514/180, 181: 552/653,510,512; 549/41 (75) Inventors: Gerardo Burton, Prov. de Buenos See application file for complete search history. Aires (AR); Carlos P. Lantos, Buenos Aires (AR); Adriana Silvia Veleiro, (56) References Cited Martinez (AR) U.S. PATENT DOCUMENTS (73) Assignee: Applied Research Systems ARS Holding N.V., Curacao (NL) 6,303,591 B1 * 10/2001 Burton et al. ............... 514f179 FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 EP O 348 910 1, 1990 U.S.C. 154(b) by 148 days. EP O 903 146 3, 1999 OTHER PUBLICATIONS (21) Appl. No.: 10/363,860 “Synthesis of 21-hydroxy-11, 19-oxidopregn-4-ene-320 (22) PCT Filed: Sep. 17, 2001 dione and 21-hydroxy-6, 19-oxidopregn-4-ene-320-dione': Steroids vol. 60, No. 3, pp. 268-271, 1995.* (86). PCT No.: PCT/EPO1/10750 (Continued) S 371 (c)(1), (2), (4) Date: Aug. 20, 2003 Primary Examiner Sabiha Qazi (74) Attorney, Agent, or Firm Oblon, Spivak, McClelland, (87) PCT Pub. No.: WO02/22647 Maier & Neustadt, P.C. PCT Pub. Date: Mar. 21, 2002 (57) ABSTRACT (65) Prior Publication Data The present invention is related to novel 21-hydroxy-6.19 US 2004/002984.6 A1 Feb. -

The Σ1 Receptor Engages the Redox-Regulated HINT1 Protein to Bring Opioid
Page 1 of 110 Original Research Communication The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control María Rodríguez-Muñoz1, Pilar Sánchez-Blázquez1, Raquel Herrero-Labrador1, Ricardo Martínez-Murillo1, Manuel Merlos2, José Miguel Vela2, Javier Garzón1 1Cajal Institute, Consejo Superior de Investigaciones Científicas (CSIC), Avenida Doctor Arce, 37. 28002 Madrid, Spain. 2Drug Discovery & Preclinical Development, Esteve. Scientific Park of Barcelona, Bardiri y Reixac 4-8, 08028, Barcelona, Spain. Running head: σ1R engages NMDAR control on MOR Correspondence to: Javier Garzón. Neurofarmacología, Instituto Cajal, Avenida Doctor Arce 37, 28002 Madrid, Spain. Tel: 34 91 5854733, Fax: 34 91 5854754. E- mail: [email protected] Antioxidants & Redox Signaling Word count: 6950 Reference number: 70 Grayscale Illustrations: Figures 5, 6, 8 and 9 Color Illustrations: Figures 3 and 4 Color online, B&W print: Figures 1, 2, 7, and 10 The 1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA negative control (doi: 10.1089/ars.2014.5993) This article has been peer-reviewed and accepted for publication, but yet to undergo copyediting proof correction. The final published version may differ from this proof. 1 Page 2 of 110 2 Abstract Aims: The in vivo pharmacology of the sigma 1 receptor (σ1R) is certainly complex; however, σ1R antagonists are of therapeutic interest because they enhance mu-opioid receptor (MOR)-mediated antinociception and reduce neuropathic pain. Thus, we investigated whether the σ1R is involved in the negative control that glutamate N- methyl-D-aspartate receptors (NMDARs) exert on opioid antinociception. -

Pregn-5-Ene-3,20-Dione, Pregnenolone and Related Progestins on Ovulation in Pmsg-Treated Immature Rats
EFFECT OF 5\m=infty\-DIHYDROPROGESTERONE, PREGN-5-ENE-3,20-DIONE, PREGNENOLONE AND RELATED PROGESTINS ON OVULATION IN PMSG-TREATED IMMATURE RATS B. N. SRIDHARAN, R. K. MEYER and H. J. KARAVOLAS Departments of Zoolog? and Physiological Chemistry, The Endocrinology-Reproductive Physiology Program, University of Wisconsin, Madison, Wisconsin 53706, U.S.A. (Received 9th January 1973) Summary. The facilitative effects of certain progestational steroids on ovulation were investigated by using 25-day-old female rats which had been treated on Day 22 with a non-ovulatory dose of PMSG (12 i.u.). Ovulation was caused by treatment with pregnenolone or progesterone on the morning of Day 24. The dose of pregnenolone required was higher than that ofprogesterone. Progesterone had this effect throughout the morning of Day 24, while prenenolone was only effective between 07.00 and 10.00 hours on Day 24. The two metabolites of progesterone in the hypothalamus and uterus, 5\g=a\-dihydroprogesteroneand 3\g=a\\x=req-\ hydroxy-5\m=infty\-pregnan-20-one,were also tested for their ability to cause ovulation. Although 5\g=a\-dihydroprogesteronepossessed this ability, a dose three to four times that of progesterone was required to produce a comparable effect. Doses of up to 1 \m=.\5mg 3\g=a\-hydroxy-5\g=a\-pregnan-20-one were without effect. Neither 17\g=a\-hydroxypregnenolonenor 17\g=a\-hydroxy- progesterone had any effect in influencing ovulation. Pregn-5-ene-3,20-dione had a facilitative ability comparable to that of progesterone in doses of 0\m=.\25mg or higher. -

Pregnenolone in the Treatment of Rheumatoid Arthritis by G
Ann Rheum Dis: first published as 10.1136/ard.10.1.32 on 1 March 1951. Downloaded from PREGNENOLONE IN THE TREATMENT OF RHEUMATOID ARTHRITIS BY G. NORMAN MYERS Regional Medical Research Centre, Royal Bath Hospital, Harrogate Since Hench, Kendall, Slocumb, and Polley (1949) described their results from the administration of cortisone (17-hydroxy-1 1-dehydrocorticosterone) and of the anterior pituitary adrenocorticotrophic hormone in the treatment of rheumatoid arthritis, there has been much speculation regarding the possible use of other substances which might produce similar effects. The search for such substances has been confined primarily to tWo main groups: (a) those, like adrenaline, which are known to stimulate the production of the anterior pituitary corticotrophic hormone, (b) those which ,may be precursors of more active steroid hormones. copyright. From the chemical standpoint A-5-pregnenolone has been suggested as a precursor and has attracted the attention of a number of workers. Davison and others (1950) treated thirty arthritis patients with pregnenolone acetate, fourteen of whom were suffering from rheumatoid arthritis. Solutions in oil, as well as aqueous suspensions, were administered by the intramuscular route. Daily injections, up to 200 and 300 mg. respectively, were given over a http://ard.bmj.com/ period of 3 to 8 weeks. They reported results which appear to be definitely favourable. Pain and stiffness in joints was diminished, muscle strength was increased, and fatigue was less evident. A feeling of " well-being " was estab- lished, usually within a few days. Reduction in joint swelling was slow, and reduction in the erythrocyte sedimentation rate, if any, did not run parallel with the clinical improvement, but followed it. -

Nicolet Standard Collection of FT-IR Spectra
Nicolet Standard Collection of FT-IR Spectra Library Listing – 3,119 spectra This collection is suited to the needs of analytical services and investigational laboratories. It provides a wide array of common chemicals in a lower cost package and can be used in combination with the Nicolet Standard Collection of FT-Raman Spectra to provide analysts a higher degree of confidence when identifying unknowns. The Nicolet Standard Collection of FT-IR Spectra includes 3,119 spectra of common chemicals with representatives of major functional groups and combinations of functional groups. It contains the same chemical series available in the Nicolet Standard Collection of FT-Raman Spectra. These matched libraries can be used alone or in combination with Thermo Fisher’s unique OMNIC Linked Search software. Nicolet Standard Collection of FT-IR Spectra Index Compound Name Index Compound Name 1177 (+)-1,4-Androstadiene-3,17-dione 1498 (+/-)-Ketamine .HCl; 2-(2- 140 (+)-11a-Hydroxyprogesterone, 95% Chlorophenyl)-2- 1221 (+)-2'-Deoxyuridine, 99+% (methylamino)cyclohex 2999 (+)-2,3-O-Benzylidene-D-threitol 1104 (+/-)-Octopamine .HCl, 98% 1291 (+)-2-Phenyl-1-propanol, 97% 2541 (+/-)-Pantothenol 933 (+)-3-Nitro-L-tyrosine, 99% 2058 (+/-)- 833 (+)-4-Cholesten-3-one Tetracyclo[6.6.2.0(2,7).0(9,14)]hexadec 739 (+)-6-Aminopenicillanic acid, 96% a-2,4,6,9,11,13-he 2361 (+)-Arabinogalactan 579 (+/-)-a-Benzoin oxime, 99% 2545 (+)-Boldine .HCl; 1,10- 2290 (+/-)-cis-2-Methylcyclohexanol, 97% Dimethoxyaporphine-2,9-diol .HCl 2287 (+/-)-trans-1,2-Dibromocyclohexane, -

Comparison of Pregnenolone Sulfate, Pregnanolone and Estradiol Levels
Rustichelli et al. The Journal of Headache and Pain (2021) 22:13 The Journal of Headache https://doi.org/10.1186/s10194-021-01231-9 and Pain SHORT REPORT Open Access Comparison of pregnenolone sulfate, pregnanolone and estradiol levels between patients with menstrually-related migraine and controls: an exploratory study Cecilia Rustichelli1, Elisa Bellei2, Stefania Bergamini2, Emanuela Monari2, Flavia Lo Castro3, Carlo Baraldi4, Aldo Tomasi2 and Anna Ferrari4* Abstract Background: Neurosteroids affect the balance between neuroexcitation and neuroinhibition but have been little studied in migraine. We compared the serum levels of pregnenolone sulfate, pregnanolone and estradiol in women with menstrually-related migraine and controls and analysed if a correlation existed between the levels of the three hormones and history of migraine and age. Methods: Thirty women (mean age ± SD: 33.5 ± 7.1) with menstrually-related migraine (MM group) and 30 aged- matched controls (mean age ± SD: 30.9 ± 7.9) participated in the exploratory study. Pregnenolone sulfate and pregnanolone serum levels were analysed by liquid chromatography-tandem mass spectrometry, while estradiol levels by enzyme-linked immunosorbent assay. Results: Serum levels of pregnenolone sulfate and pregnanolone were significantly lower in the MM group than in controls (pregnenolone sulfate: P = 0.0328; pregnanolone: P = 0.0271, Student’s t-test), while estradiol levels were similar. In MM group, pregnenolone sulfate serum levels were negatively correlated with history of migraine (R2 = 0.1369; P = 0.0482) and age (R2 = 0.2826, P = 0.0025) while pregnenolone sulfate levels were not age-related in the control group (R2 = 0.04436, P = 0.4337, linear regression analysis). -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

Pregnenolone, a Fruit of Cholesterol
PREGNENOLONE, A FRUIT OF CHOLESTEROL Mother of Progesterone & D.H.E.A. The following information comes from Dr. Ray Peat, who has done pioneering research on the anti-aging steroids, pregnenolone, progesterone and DHEA (Dehydroepiandrosterone). I have included excerpts from his writings plus the results of interviews. Research references are provided when available, but in many cases, I could only describe the group of researchers who did the experiment. My purpose for this article is not to start a riot but to illustrate why I think it’s dangerous to artificially inhibit cholesterol formation in your body with drugs and synthetic foods. Dr. Peat accidentally discovered the effects of pregnenolone when he took some vitamin E containing a residue of pregnenolone that was left over from an experiment in solubility. Peat had been suffering from a variety of complaints, including " inflammation of the arteries, dental abscesses, asthma, migraines, and colitis ." When he took the vitamin E containing some pregnenolone in the vitamin E, he crawled out of his sick bed, took a pinch of pure pregnenolone and felt immediately better. All of his symptoms gradually disappeared and in ten weeks, his appearance changed. Many aging characteristics, such as sagging skin, "chicken neck," bags under the eyes, etc. receded. These changes were dramatically reported in a passport photo, taken one year before pregnenolone and another one taken 10 weeks after pregnenolone therapy was initiated. I regret that the original photos are not available for inclusion here. When I saw this photo, presented in one of his newsletters, I fell off my chair, dashed to the phone and called Dr. -

Neurosteroids: Pregnenolone in Human Sciatic Nerves
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 6790-6793, August 1992 Neurobiology Neurosteroids: Pregnenolone in human sciatic nerves (dehydroepiandrosterone/mass spectrometry/steroid sulfates/steroid fatty acid esters) ROBERT MORFIN*t, JACQUES YOUNG*, COLETTE CORPtCHOT*, BORJE EGESTADt, JAN SJOVALLt, AND ETIENNE-EMILE BAULIEU*§ *Institut National de la Santd et de la Recherche MWdicale U33, Laboratoire Hormones, 94276 Bicetre Cedex, France; and tDepartment of Physiological Chemistry, Karolinska Institutet, Stockholm, Sweden Contributed by Etienne-Emile Baulieu, March 30, 1992 ABSTRACT The characterization and quantification of Tissues. Tissues were obtained <48 hr after death from pregnenolone in human sciatic nerves were undertaken, fol- cadavers of both sexes bequeathed to medical research. lowing previous demonstration of the synthesis of this steroid Corpses were kept refrigerated at 40C. Ages ranged from 44 in rat brain oligodendrocytes, to explore the hypothesis that to 90 years. Causes of death were not recorded. Portions of Schwann cells may demonstrate the same biosynthetic activity. sciatic nerves, tendons, and muscle were taken close to the Pregnenolone was definitively identified by mass spectrometry knee. Samples of spleen were obtained in one case. The and quantified by specific radioinmunoassay. Its concentra- collected samples (0.675-4.870 g) were first weighed and then tion (mean ± SD, 63.9 ± 45.9 ng/g of wet tissue, n = 12) was frozen in liquid nitrogen until processed further. 2100 times the plasma level and concentration found in Extraction and Purification Procedures. Frozen samples in tendons and muscle. No correlation was found with sex or age. liquid nitrogen were first pulverized in a mortar and then Free dehydroepiandrosterone as well as sulfate and fatty acid homogenized in phosphate-buffered saline at 40C. -
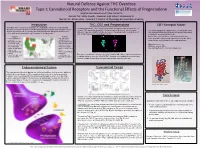
Introduction Endocannabinoid System Cannabinoid Tetrad THC, CB1 And
Natural Defense Against THC Overdose Type-1 Cannabinoid Receptors and the Functional Effects of Pregnenolone Brigitte Rios Llamosa and Alexis Camacho Advisor: Mr. Justin Spaeth, Messmer High School, Milwaukee WI Mentor: Dr. Aaron Miller - Assistant Professor of Physiology at Concordia University Introduction THC, CB1 and Pregnenolone CB1 Receptor Model Cannabis, often referred to as marijuana, is a drug produced from the Cannabis plant. Tetrahydrocannabinol (THC), the active ingredient in marijuana, also activates the CB1 Recently, marijuana has become an often debated topic as people work to legalize its use receptor. THC and similar drugs have therapeutic potential in the treatment of pain, Our model highlights the amino acids E133 and R409, which for both recreational (as in Colorado) and medical purposes. Marijuana is able to help Alzheimer’s disease, anxiety, arthritis, and cancer. A downside to the medicinal use of form hydrogen bonds with pregnenolone and are required for relieve pain, but it can also lower performance in everyday tasks. THC is that it also induces psychotropic effects. its binding to the allosteric site of CB1. Negatives Positives Both of these amino acids are colored in cpk color and • Poor coordination of • Can help control connected with a strut to our pregnenolone molecule. Other movement epileptic seizures areas that are highlighted are color-coded as follows: • Afterwards, users feel • May decrease anxiety tired or depressed • Can slow progression Alpha helixes are red. • Increases heartbeat and of diseases such as risk of heart attack Alzheimer’s disease Struts are colored white. • Inability to understand • Has been used to treat Non-motif portions are colored cornflower blue.