Academy Actions Featured Fellow Fellow Vaccine Efforts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View Curriculum Vitae
(updated 3/2018) Curriculum Vitae Sarah Wackerbarth, Ph.D. 105B College of Public Health Building 111 Washington Avenue Lexington, Kentucky, 40536-0003 859/218-2079 [email protected] PROFESSIONAL EXPERIENCE 7/2009 – current Associate Professor (with tenure), Health Management and Policy, College of Public Health, University of Kentucky 6/2009 – 3/2010 Visiting Research Associate, Center for Health Enhancement Systems Studies, University of Wisconsin 2003 – 9/2008 Director of Graduate Studies – Masters of Health Administration, University of Kentucky 2002 – 6/2009 Associate Professor (with tenure), Martin School of Public Policy and Administration, University of Kentucky 1997 – 2002 Assistant Professor, Martin School of Public Policy and Administration and Gerontology, University of Kentucky 1997 – present Research Associate, Sanders-Brown Center on Aging, University of Kentucky 1996 – 1997 Project Director and Developer, Alzheimer’s-CHESS, Center for Health Systems Research and Analysis, University of Wisconsin - Madison 1992 – 1995 Teaching & Research Assistant, Department of Industrial Engineering, University of Wisconsin - Madison 1992 – 1995 Research Assistant, Center for Health Systems Research and Analysis, University of Wisconsin - Madison 1990 – 1992 Management Engineer, University of Wisconsin Hospital and Clinics, Madison, Wisconsin EDUCATION 1997 Ph.D., University of Wisconsin - Madison Industrial Engineering: Operations Research/Decision Science Minor: Statistics Major Professor: David H. Gustafson, Ph.D. Dissertation: Modeling -

CURRICULUM VITAE CARMEN TRUITT AGOURIDIS, Ph.D., P.E
June 9, 2020 Carmen T. Agouridis, PhD, PE, MPP, MBA CURRICULUM VITAE CARMEN TRUITT AGOURIDIS, Ph.D., P.E., M.P.P. EXTENSION ASSOCIATE PROFESSOR UNIVERSITY OF KENTUCKY CURRENT POSITION ................................................................................................................................ 1 RESEARCH, TEACHING & EXTENSION INTERESTS ................................................................................... 1 EDUCATION ............................................................................................................................................. 1 CERTIFICATION ........................................................................................................................................ 2 PROFESSIONAL EXPERIENCE ................................................................................................................... 2 AWARDED GRANTS ................................................................................................................................. 4 HATCH PROJECTS .................................................................................................................................. 14 GRANTS IN REVIEW ............................................................................................................................... 14 NON-AWARDED GRANTS ...................................................................................................................... 14 NATIONAL ACADEMIES OF SCIENCE, ENGINEERING, AND MEDICINE ................................................. -

2007 Advances in Pharmacy Practice Annual Fall Conference - Registration Form Registration - Conference Fall Annual Practice Pharmacy in Advances 2007
Lexington, Kentucky Lexington, 1375 Harrodsburg Road Harrodsburg 1375 The Crowne Plaza Campbell House Campbell Plaza Crowne The October 26-28, 2007 26-28, October 2007 Sponsored by Sponsored Advances in Pharmacy Practice Annual Fall Conference Fall Annual Annual Fall Conference Advances in Pharmacy Practice Pharmacy in Advances 2007 Meeting Facilities and Accommodations All sessions will be held at The Crowne Plaza Campbell House Inn, 1375 Harrodsburg Road, Lexington, KY 40504 (800) 354-9235. A block of sleeping rooms has been reserved at the discounted rate of $119 per night single/double occupancy plus tax. Reservation deadline is October 4, 2007. Reservations made after that date will be on a space/rate available basis. Be sure to mention you are attending the UK College of Pharmacy CE program to receive the conference rate. Visit http://www.thecampbellhouse.net for complete hotel information and directions. An Equal Opportunity University Opportunity Equal An Football Tickets The Kentucky Wildcats will be hosting the Mississippi State Bulldogs for Homecoming on October 27. Game time is tentatively set for 7:00 PM. Fall Conference attendees may purchase two (2) tickets per registrant. Cost is $30.00 per ticket and must be prepaid by October 12, 2007. Please send a separate check made payable to UKAA. Football tickets must be paid with a check. No other form of payment will be accepted. Special Services The University of Kentucky provides reasonable accommodation or special diet with adequate notice. Please indicate need on the registration form or call Deloris Mercer at (859) 257-5320 ext. 80337 by October 19, 2007, in order to ensure that adequate arrangements are made. -

FCR 18 Capital Construction Report
FCR 18 Office of the President December 15, 2020 Members, Board of Trustees: CAPITAL CONSTRUCTION REPORT Recommendation: that the capital construction report for the three months ending September 30, 2020 be accepted. This report refers only to projects that had activity within this quarter. Background: Under House Bill 622 enacted in the 1982 session of the Kentucky General Assembly, the university is authorized to enter into architectural, engineering and related consultant contracts for the purpose of accomplishing capital construction at the University of Kentucky. For the period July 1, 2020 thru September 30, 2020: There were five new contracts this quarter: Project 2402.9 Renovate/Upgrade UK HealthCare Facilities (Phase I-I) – Fit-up 5th Floor Pavilion A - Turner Construction Company, $2,125,895 (Construction) Project 2511.8 Renew/Modernize Facilities Capital Project (Frazee Hall) - Lord, Aeck, & Sargent, $1,131,679 (Design) Project 2524.0 Construct/Improve Greek Housing (Alpha Delta Pi) Capital Project - Congleton-Hacker Company, $4,852,019 (Construction) Project 2526.0 Construct Beam Institute I Capital Project - Joseph & Joseph Architects, $388,207 (Design) Project 2538.0 Construct Research Building (Fit-Up Two Wet Labs) Capital Project - Whiting-Turner Contracting Company, $689,700 (Construction) Five contracts were completed this quarter: Project 2444.0 Expand/Renovate/Upgrade Law Building Capital Project - Congleton-Hacker Company, $45,061,784 Project 2457.0 Renovate/Improve Clinical/Ambulatory Services Capital Project (Brachytherapy) -

Wildcat Ct R
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 A rl Park Ave in Marquis Ave g to n A Eldemere Rd Tates Creek Rd P v P e K Transylvania Park r a D st t le Baldwin Ave e e R Kentucky Ct s Beaumont Ave d Av n Melrose Ave Stone Ave Oldham Ave d u ar S h rc O Tremont Ave l P n E. Vine St Aylesford Pl Su i O O UK Training m k mi e e t D e v Woodland Ave r Resource Center A Mt Vernon Dr m 1 Quality Street lid c c M u E Linden Walk Berry Ln Cottage Grove Ln Lexington Ave Hagerman Ct Oldham Ct N Kastle Rd N 240 495 Providence Rd Club S B " ) . Cliff Hagan M ShivelyField Track Stadium and Sports 633 506 d E. High St a 314 D Field rt n r Baseball R in w t o Stadium n L 488 st 344 Farmhouse r o u B " ) t Fraternity e 135 136 283 John W. Cropp h -opening p m e E. Maxwell St 432 o h r 343 o 130 c M M K January 2014 C B " ) Field 345 Scoville Rd d e i 238 r n 315 e g D R 644 B " ) B 23 r B 119 121 Cooperstown i e la e lv r c t d Woodland Glen I and II Apartments t Club 507 n Residence Halls n Sports i 604 o a 494 - opening August 2014 132 113 Tateswood Dr 131 M Fields R " ) 611 B Rose Ln B " ) 452 d O n R 19 Penn Ct 9925 Bimini Rd l d o bbi L 433 566 D L Penn Ave 565 451 Lexington Ave 127 567 Ronald 8633 505 122 277 KET McDonald B " ) Hope Columbia Ave House Lodge 15 128 241 21 B " ) Sp 129 126 504 orts " ) B " ) B Champions 149 Ce W B " ) n " t 11 Court I and II Avenue Of Champions V er Soccer Fields ResidenceHalls 67 !( D 12 r - opening 456 V Ar !( K 613 B " ) 125 b K August 2014 Stoll Field r o 22 68 572 r 9 -
March/April: Behavioral Health
MEDICAL NEWS THE BUSINESS OF HEALTHCARE SERVING KENTUCKY AND SOUTHERN INDIANA $2.50 MARCH/APRIL 2021 News in Brief page 2 / People in Brief page 4 / Commentary page 18 Physician Spotlight Meet David Austin Hudson, MD, with RACIAL TRAUMA New Vista Community AND Mental Health THE Center Read more on page 5 HUDSON UK, partners break ground on new Coldstream Research Lab By Sally McMahon This training included didactic lectures, readings Read more on page 14 Steven Kniffley Jr., PsyD, is a on Black psychology, clinical psychologist who leads Spald- Steven Kniffley Jr.: My goals for Universal Medical ing University’s Collective Care Cen- Liberation psychology and the first year in- Supply offers ter – one of the nation’s only behavioral the assessment and treatment clude coordinating American made PPE health clinics to specialize in treating and implementing of racial trauma in various race-based trauma and stress. a university-wide Since 2018, he has served as associate racial and ethnic groups, and cultural climate as- director of Spalding’s Center for Behavior- sessment as well as viewing videos demonstrating al Health training clinic, of which the Col- developing a diver- lective Care Center is a specialty division. racial trauma assessment and sity, equity, justice, Kniffley’s area of expertise is re- and inclusion stra- therapy techniques.” search and clinical work with Black KNIFFLEY tegic plan. males. Specifically, his work focuses on Read more on page 16 understanding and developing culturally cifically, racial trauma can contribute MN: What is racial trauma? appropriate interventions for Black male to mental health difficulties related SK: Trauma that stems from the re- psychopathology as well as barriers to to poor concentration, flashbacks and sult of chronic experiences racism and dis- academic success for this population. -
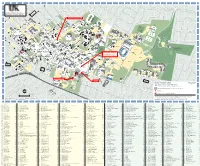
Parking Garage Pedway Approximate Location of Auditorium on 1St Floor Of
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 A rl P in a M g rk a to r n A q e v e u A e v is v E Tates Creek Rd v A A ld P e A e P T in v e K m r w e r s a er a d D o e s e l v t n a t lr le R s A d y B e e e R lv K v s B t d a e A n e M n S n n O d u a o to i t l r S u t n a u d a m m S g r P c h h o e r e c n r n e k a r l n e A i t a y m t A T D n n n t r O L i v i e k C v P V a e A e n e r C e t e . r o n T v i e t n S v E c S A e r K r y um k o O O U u y e r o lit le m e s V i e G s a e t e u fo v W t Dr R Q r A M n m e d o g 1 id L c P l o a c d y M t l u l m r t E a & r o n iu e d L k d d B R C in A c a d a t e L v r S c e H e e T x a n d n l e in g W C O e g C e ld i id K N t rm a h F v a N o t lk 2 a o s n a 5 4 m r tle t A n 49 0 R S v C P d h S e t ig . -

Spring 2016 (Pdf)
SPECIAL EDITION 2016 Step STRIKING IN THE PURSUIT OF NURSING EXCELLENCE, UK HEALTHCARE ACHIEVES THE GOLD STANDARD OF NURSING CARE— ANCC MAGNET RECOGNITION® In Step for the Commonwealth Dear Colleagues, We find it hard to believe this is our seventh edition of In Step!Our journey began in 2010 when we decided to showcase and honor the rich experiences of UK nurses and create a forum for telling our stories of collaboration among interdisciplinary teams and also the invaluable partnership between the UK College of Nursing and practicing nurses at UK HealthCare®. As you explore this edition, we hope you can experience the energy, enthusiasm and optimism emanating from our teams. Dr. Heath is in her second year as the dean of the College of Nursing and has brought so much energy and passion to the faculty and students—the excitement is palpable. This mood translates to our nursing staff, many of whom are pursuing baccalaureate and graduate degrees at the College of Nursing. By the time you read this, we will have celebrated one of our major achievements—being designated once again as a Magnet system. It has been a long and sometimes difficult journey, but the benefits for our clinical, patient and staff experience outcomes and practice model maturation have been worth the navigation of our pathway. The interdisciplinary teams of practicing professionals and learners at UK HealthCare are so special. Our nursing practice is vibrant and resonates across the health system. We hope that in these pages you will feel the sense of accomplishment experienced by our teams each and every day. -

House Director Resource Manual 2018-2019
House Director Resource Manual 2018-2019 Issued by Fraternity and Sorority Life 518 Patterson Office Tower University of Kentucky Lexington, KY 40506-0027 www.uky.edu/GreekLife [email protected] @UKYGreek (859) 257-3151 FAX (859) 323-1525 Emergency Phone Numbers Name Phone Fall 2018 Chapter Advisor _______________________________ ____________ Chapter President _______________________________ ____________ Chapter House Manager _______________________________ ____________ Spring 2019 Chapter Advisor _______________________________ ____________ Chapter President _______________________________ ____________ Chapter House Manager _______________________________ ____________ Susan West University Police Director of Fraternity and Sorority Life 859-257-1616 [email protected] 859-338-8222 Lexington Police 859-258-3600 Emily Britt Assistant Director Hospital Emergency Room Panhellenic Council Advisor 859-233-5901 [email protected] Fire Marshals Jenna Lyons University of Kentucky Assistant Director Jason Ellis NPHC & UGC Advisor [email protected] [email protected] 859-257-6326 Lexington Will Takewell Capt. Marcus Blanton Assistant Director [email protected] Interfraternity Council Advisor 859-231-5686 [email protected] Robert Smith House Director Coordinator 814.572.8030 [email protected] Table of Contents Fraternity and Sorority Life University of Kentucky Fraternity and Sorority Life Office All Chapter Housing Status 1 FSL Terminology 3 Mission & Focus 6 Important Dates 8 Chapter Officer Training Series 10 House Director -

The UK Healthcare Onboarding Campus Navigation Guide Covers
The UK Healthcare Onboarding Campus Navigation Guide covers common destinations for new hires through completion of UK Healthcare New Employee Orientation and/or the first week as an employee, Please consult with your manager or Transportation Services about parking and transportation to your normal work location. For additional training locations, please use the University of Kentucky’s interactive campus map at https://maps.uky.edu/campusmap. Chandler Campus Locations (numbers correspond with map): 1. Pre-Employment Screening Office. Location to initiate the pre-employment screening process. Parking is available behind the house. Address: 1101 S. Limestone, Lexington, KY 40506. 2. University Health Service Building (UHS). Location of pre-employment screening and the Employee Health visit. Address: 830 S. Limestone, Lexington, KY 40536. 3. Scovell Hall. Central Human Resources office; location for completing I-9 Employment Eligibility Verification forms, and location of Benefits Customer Service. Metered parking is available from Huguelet Drive. Address: 670 S. Limestone, Lexington, KY 40546. 4. Parking and Transportation Office. Located on the ground floor of the Press Avenue parking garage. Metered parking spots are available on the street and near the entrance to the structure. For questions, call 859-323- 1212. Address: 721 Press Avenue, Lexington, KY 40506. 5. Orange Lot at Kroger Field. Location where most employees will park and take the Orange Route Shuttle if the work location is on the Chandler campus. The Orange Lot is located on University Drive directly across the street from the Kroger Field stadium. A pole indication ‘Orange Lot’ is located at each entrance to the lot. Address: 1540 University Drive. -

The Power of Advanced Medicine Propels Us
uk healthcare THE POWER OF 2016 ADVANCED annual report annual MEDICINE 2016 ANNUAL REPORT 2016 ANNUAL REPORT CONTENTS Executive Summary 2 University Health Care Committee 6 The UK HealthCare Brand 7 Quality of Care 8 Kentucky Health Collaborative 12 UK College of Medicine 2016 Annual Report 14 Statistics & Financials 26 Philanthropy 38 UK Arts in HealthCare 40 On the cover: John D’Orazio, MD, (left) treats pediatric cancer patients and specializes in research on melanoma. Sheena Hall, RN, (right) works in 4 East at UK Good Samaritan Hospital. A REPORT ON FISCAL YEAR 2016 FROM THE EXECUTIVE VICE PRESIDENT FOR HEALTH AFFAIRS THE POWER OF ADVANCED MEDICINE PROPELS US Fiscal year 2016 was one of high levels of activity on all In 2016, we began a serious turn toward true fronts as we organized the clinical enterprise to execute interdisciplinary service lines. Service lines bring on our 2015-2020 Strategic Plan. Despite the highly people together; specialists of all kinds who focus on volatile nature of today’s health care environment, our a set of patients. Such a highly connected, interrelated strategic vision at its core remains unchanged – a focus team is necessary to care for complex patients. on advanced subspecialty care, building relationships and quality improvements. Stronger Relationships Our second longstanding strategy has been to build Advanced Medicine relationships with other providers. Our relationships In 2003, we made a strategic decision to focus on have evolved into networks for cancer, heart and stroke. advanced specialty care. That decision changed the In 2016, several of these relationships culminated in medical center’s trajectory – away from the “safety the Kentucky Health Collaborative – a group of 10 net” hospital we might have become and toward hospital systems with statewide coverage representing teams of specialists and subspecialists collaborating more than 50 hospitals. -
Program - About a Child and Youth Violence, Bullying, and Abuse, Prevention/Health Education Curriculum “Survive & Thrive Skills Training”
YouthAlert! (YA!) Program - About A Child and Youth Violence, Bullying, and Abuse, Prevention/Health Education Curriculum “Survive & Thrive Skills Training” YouthAlert! (YA!) U.S.A. Board Members and Officers Douglas A. Wain, Chief Executive Officer/Executive Director, YouthAlert! (YA!) U.S.A., 2711 Barbados Lane, Lexington, KY, 40509-9508, (859) 494-3677, [email protected] Elisa Wain, Educator, Fayette County Public Schools, Lexington, KY, 2711 Barbados Lane, Lexington, KY, 40509-9508, (859) 494-3789, [email protected] _________________________________________________________________________________________ © YouthAlert! (YA!) U.S.A. 2018 859.494.3677 www.youthalert.us [email protected] Page 1 of 4 YouthAlert! (YA!) Program - About A Child and Youth Violence, Bullying, and Abuse, Prevention/Health Education Curriculum “Survive & Thrive Skills Training” Diane Turner Minnifield, Fayette County Attorney's Office, 110 W. Vine Street Lexington, KY, 40507, (859) 254-4941, [email protected] “YouthAlert! (YA!) allows us adults to figure out way to help kids stop this senseless and useless violence YouthAlert! (YA!) provides you with an opportunity raise your concerns and meet halfway, to figure out other ways of handing conflict that doesn’t require violence.” Stuart Silberman, Former Executive Director, Prichard Committee for Academic Excellence, Former Superintendent, Fayette County Public Schools, Lexington, Kentucky, (859) 229-8040, [email protected] “I am a founding board member of this organization and have watched it impact many, many students in preventing violence. The evaluations that come from the training are remarkably positive. The administrators and teachers at the schools where the program has been implemented agree that this makes a difference. The students also agree.” _________________________________________________________________________________________ © YouthAlert! (YA!) U.S.A.