Computational Modeling of Skull Bone Structures and Simulation of Skull Fractures Using the YEAHM Head Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
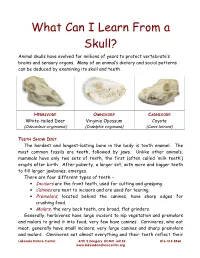
What Can I Learn from a Skull? Animal Skulls Have Evolved for Millions of Years to Protect Vertebrate’S Brains and Sensory Organs
What Can I Learn From a Skull? Animal skulls have evolved for millions of years to protect vertebrate’s brains and sensory organs. Many of an animal’s dietary and social patterns can be deduced by examining its skull and teeth. HERBIVORE OMNIVORE CARNIVORE White-tailed Deer Virginia Opossum Coyote (Odocoileus virginianus) (Didelphis virginiana) (Canis latrans) TEETH SHOW DIET . The hardest and longest-lasting bone in the body is tooth enamel. The most common fossils are teeth, followed by jaws. Unlike other animals, mammals have only two sets of teeth, the first (often called ‘milk teeth’) erupts after birth. After puberty, a larger set, with more and bigger teeth to fill larger jawbones, emerges. There are four different types of teeth – • Incisors are the front teeth, used for cutting and grasping. • Canines are next to incisors and are used for tearing. • Premolars , located behind the canines, have sharp edges for crushing food. • Molars , the very back teeth, are broad, flat grinders. Generally, herbivores have large incisors to nip vegetation and premolars and molars to grind it into food; very few have canines. Carnivores, who eat meat, generally have small incisors, very large canines and sharp premolars and molars. Omnivores eat almost everything and their teeth reflect their Lakeside Nature Center 4701 E Gregory, KCMO 64132 816-513-8960 www.lakesidenaturecenter.org preferences; they have all four types of teeth. In fact, if you’d like to see an excellent example of an omnivore’s teeth, look in the mirror. EYE PLACEMENT IDENTIFIES PREDATORS . Carnivores generally have large eyes, placed so that the eyes look forward and the areas of vision of the two eyes overlap. -

Cervical Spine Injury Risk Factors in Children with Blunt Trauma Julie C
Cervical Spine Injury Risk Factors in Children With Blunt Trauma Julie C. Leonard, MD, MPH,a Lorin R. Browne, DO,b Fahd A. Ahmad, MD, MSCI,c Hamilton Schwartz, MD, MEd,d Michael Wallendorf, PhD,e Jeffrey R. Leonard, MD,f E. Brooke Lerner, PhD,b Nathan Kuppermann, MD, MPHg BACKGROUND: Adult prediction rules for cervical spine injury (CSI) exist; however, pediatric rules abstract do not. Our objectives were to determine test accuracies of retrospectively identified CSI risk factors in a prospective pediatric cohort and compare them to a de novo risk model. METHODS: We conducted a 4-center, prospective observational study of children 0 to 17 years old who experienced blunt trauma and underwent emergency medical services scene response, trauma evaluation, and/or cervical imaging. Emergency department providers recorded CSI risk factors. CSIs were classified by reviewing imaging, consultations, and/or telephone follow-up. We calculated bivariable relative risks, multivariable odds ratios, and test characteristics for the retrospective risk model and a de novo model. RESULTS: Of 4091 enrolled children, 74 (1.8%) had CSIs. Fourteen factors had bivariable associations with CSIs: diving, axial load, clotheslining, loss of consciousness, neck pain, inability to move neck, altered mental status, signs of basilar skull fracture, torso injury, thoracic injury, intubation, respiratory distress, decreased oxygen saturation, and neurologic deficits. The retrospective model (high-risk motor vehicle crash, diving, predisposing condition, neck pain, decreased neck mobility (report or exam), altered mental status, neurologic deficits, or torso injury) was 90.5% (95% confidence interval: 83.9%–97.2%) sensitive and 45.6% (44.0%–47.1%) specific for CSIs. -

The Dynamic Impact Behavior of the Human Neurocranium
www.nature.com/scientificreports OPEN The dynamic impact behavior of the human neurocranium Johann Zwirner1,2*, Benjamin Ondruschka2,3, Mario Scholze4,5, Joshua Workman6, Ashvin Thambyah6 & Niels Hammer5,7,8 Realistic biomechanical models of the human head should accurately refect the mechanical properties of all neurocranial bones. Previous studies predominantly focused on static testing setups, males, restricted age ranges and scarcely investigated the temporal area. This given study determined the biomechanical properties of 64 human neurocranial samples (age range of 3 weeks to 94 years) using testing velocities of 2.5, 3.0 and 3.5 m/s in a three-point bending setup. Maximum forces were higher with increasing testing velocities (p ≤ 0.031) but bending strengths only revealed insignifcant increases (p ≥ 0.052). The maximum force positively correlated with the sample thickness (p ≤ 0.012 at 2.0 m/s and 3.0 m/s) and bending strength negatively correlated with both age (p ≤ 0.041) and sample thickness (p ≤ 0.036). All parameters were independent of sex (p ≥ 0.120) apart from a higher bending strength of females (p = 0.040) for the 3.5 -m/s group. All parameters were independent of the post mortem interval (p ≥ 0.061). This study provides novel insights into the dynamic mechanical properties of distinct neurocranial bones over an age range spanning almost one century. It is concluded that the former are age-, site- and thickness-dependent, whereas sex dependence needs further investigation. Biomechanical parameters characterizing the load-deformation behavior of the human neurocranium are crucial for building physical models 1–3 and high-quality computational simulations 4–6 of the human head to answer com- plex biomechanical research questions to the best possible extent. -

Clinical Features and Treatment of Pediatric Orbit Fractures
ORIGINAL INVESTIGATION Clinical Features and Treatment of Pediatric Orbit Fractures Eric M. Hink, M.D.*, Leslie A. Wei, M.D.*, and Vikram D. Durairaj, M.D., F.A.C.S.*† Departments of *Ophthalmology, and †Otolaryngology and Head and Neck Surgery, University of Colorado Denver, Aurora, Colorado, U.S.A. of craniofacial skeletal development.1 Orbit fractures may be Purpose: To describe a series of orbital fractures and associated with ophthalmic, neurologic, and craniofacial inju- associated ophthalmic and craniofacial injuries in the pediatric ries that may require intervention. The result is that pediatric population. facial trauma is often managed by a diverse group of specialists, Methods: A retrospective case series of 312 pediatric and each service may have their own bias as to the ideal man- patients over a 9-year period (2002–2011) with orbit fractures agement plan.4 In addition, children’s skeletal morphology and diagnosed by CT. physiology are quite different from adults, and the benefits of Results: Five hundred ninety-one fractures in 312 patients surgery must be weighed against the possibility of detrimental were evaluated. There were 192 boys (62%) and 120 girls (38%) changes to facial skeletal growth and development. The purpose with an average age of 7.3 years (range 4 months to 16 years). of this study was to describe a series of pediatric orbital frac- Orbit fractures associated with other craniofacial fractures were tures; associated ophthalmic, neurologic, and craniofacial inju- more common (62%) than isolated orbit fractures (internal ries; and fracture management and outcomes. fractures and fractures involving the orbital rim but without extension beyond the orbit) (38%). -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

In Drivers of Open-Wheel Open Cockpit Race Cars
SPORTS MEDICINE SPINE FRACTURES IN DRIVERS OF OPEN-WHEEL OPEN COCKPIT RACE CARS – Written by Terry Trammell and Kathy Flint, USA This paper is intended to explain the mechanisms responsible for production of spinal fracture in the driver of an open cockpit single seat, open-wheel racing car (Indy Car) and what can be done to lessen the risk of fracture. In a report of fractures in multiple racing • Seated angle (approximately 45°) • Lumbar spine flexed series drivers (Championship Auto Racing • Hips and knees flexed • Seated semi-reclining Cars [CART]/Champ cars, Toyota Atlantics, Indy Racing League [IRL], Indy Lights and Figure 1: Body alignment in the Indy Car. Formula 1 [F1]), full details of the crash and mechanism of injury were captured and analysed. This author was the treating physician in all cases from 1996 to 2011. All images, medical records, data available BASILAR SKULL FRACTURE Use of this specific safety feature from the Accident Data Recorder-2 (ADR), Following a fatal distractive basilar is compulsory in most professional crash video, specific on track information, skull fracture in 1999, the Head and Neck motorsport sanctions and has resulted in a post-accident investigation of damage and Support (HANS) device was introduced into dramatic reduction to near elimination of direction of major impact correlated with Indy Cars. Basilar skull fracture occurs when fatal basilar skull fractures. No basilar skull ADR-2 data were analysed. Results provided neck tension exceeds 3113.75 N forces. fractures have occurred in the IRL since the groundwork for understanding spine No data demonstrates that HANS introduction of the HANS and since 2006 fracture and forces applied to the driver in predisposes the wearer to other cervical there has been only one cervical fracture. -

Depressed Skull Fracture (Let's Talk About
In partnership with Primary Children’s Hospital Let’s Talk About... Depressed Skull Fracture B. A. Depressed skull Indentation fracture C. Concussion A depressed skull fracture happens when one or more to be removed. Before the surgery, your child’s head fragments of the skull bone are pushed inward (see is shaved so the surgery can be completely clean. Your “B” on the illustration). An indentation (A) with no child also receives some medicine to help him or her breaks in the bone happens more often in babies. relax and sleep. During surgery, small metal plates Since the brain is beneath the skull, the skull bone and screws may be used to hold the bone fragments fragments may injure the brain (C). Your child may in place. The skin over the surgical area is closed with have a bruise on the brain (called a contusion), and sutures (SOO-churs) or skin staples. a swollen black-and-blue area on the skin. What happens after the surgery? How do you treat a depressed After the surgery, your child will stay in the hospital skull fracture? for a few days. There is a lot of equipment around To find out if your child has a skull fracture, a special the bed. This helps the staff care for your child. x-ray called a CT Scan (short for computerized While your child is in the hospital, the nurses tomography) is needed. The CT scan takes pictures of frequently check your child’s temperature, pulse, your child’s brain and skull bones. blood pressure, and alertness. -

Biomechanics of Temporo-Parietal Skull Fracture Narayan Yoganandan *, Frank A
Clinical Biomechanics 19 (2004) 225–239 www.elsevier.com/locate/clinbiomech Review Biomechanics of temporo-parietal skull fracture Narayan Yoganandan *, Frank A. Pintar Department of Neurosurgery, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226, USA Received 16 December 2003; accepted 16 December 2003 Abstract This paper presents an analysis of research on the biomechanics of head injury with an emphasis on the tolerance of the skull to lateral impacts. The anatomy of this region of the skull is briefly described from a biomechanical perspective. Human cadaver investigations using unembalmed and embalmed and intact and isolated specimens subjected to static and various types of dynamic loading (e.g., drop, impactor) are described. Fracture tolerances in the form of biomechanical variables such as peak force, peak acceleration, and head injury criteria are used in the presentation. Lateral impact data are compared, where possible, with other regions of the cranial vault (e.g., frontal and occipital bones) to provide a perspective on relative variations between different anatomic regions of the human skull. The importance of using appropriate instrumentation to derive injury metrics is underscored to guide future experiments. Relevance A unique advantage of human cadaver tests is the ability to obtain fundamental data for delineating the biomechanics of the structure and establishing tolerance limits. Force–deflection curves and acceleration time histories are used to derive secondary variables such as head injury criteria. These parameters have direct application in safety engineering, for example, in designing vehicular interiors for occupant protection. Differences in regional biomechanical tolerances of the human head have implications in clinical and biomechanical applications. -

6. the Pharynx the Pharynx, Which Forms the Upper Part of the Digestive Tract, Consists of Three Parts: the Nasopharynx, the Oropharynx and the Laryngopharynx
6. The Pharynx The pharynx, which forms the upper part of the digestive tract, consists of three parts: the nasopharynx, the oropharynx and the laryngopharynx. The principle object of this dissection is to observe the pharyngeal constrictors that form the back wall of the vocal tract. Because the cadaver is lying face down, we will consider these muscles from the back. Figure 6.1 shows their location. stylopharyngeus suuperior phayngeal constrictor mandible medial hyoid bone phayngeal constrictor inferior phayngeal constrictor Figure 6.1. Posterior view of the muscles of the pharynx. Each of the three pharyngeal constrictors has a left and right part that interdigitate (join in fingerlike branches) in the midline, forming a raphe, or union. This raphe forms the back wall of the pharynx. The superior pharyngeal constrictor is largely in the nasopharynx. It has several origins (some texts regard it as more than one muscle) one of which is the medial pterygoid plate. It assists in the constriction of the nasopharynx, but has little role in speech production other than helping form a site against which the velum may be pulled when forming a velic closure. The medial pharyngeal constrictor, which originates on the greater horn of the hyoid bone, also has little function in speech. To some extent it can be considered as an elevator of the hyoid bone, but its most important role for speech is simply as the back wall of the vocal tract. The inferior pharyngeal constrictor also performs this function, but plays a more important role constricting the pharynx in the formation of pharyngeal consonants. -

The Skull O Neurocranium, Form and Function O Dermatocranium, Form
Lesson 15 ◊ Lesson Outline: ♦ The Skull o Neurocranium, Form and Function o Dermatocranium, Form and Function o Splanchnocranium, Form and Function • Evolution and Design of Jaws • Fate of the Splanchnocranium ♦ Trends ◊ Objectives: At the end of this lesson, you should be able to: ♦ Describe the structure and function of the neurocranium ♦ Describe the structure and function of the dermatocranium ♦ Describe the origin of the splanchnocranium and discuss the various structures that have evolved from it. ♦ Describe the structure and function of the various structures that have been derived from the splanchnocranium ♦ Discuss various types of jaw suspension and the significance of the differences in each type ◊ References: ♦ Chapter: 9: 162-198 ◊ Reading for Next Lesson: ♦ Chapter: 9: 162-198 The Skull: From an anatomical perspective, the skull is composed of three parts based on the origins of the various components that make up the final product. These are the: Neurocranium (Chondocranium) Dermatocranium Splanchnocranium Each part is distinguished by its ontogenetic and phylogenetic origins although all three work together to produce the skull. The first two are considered part of the Cranial Skeleton. The latter is considered as a separate Visceral Skeleton in our textbook. Many other morphologists include the visceral skeleton as part of the cranial skeleton. This is a complex group of elements that are derived from the ancestral skeleton of the branchial arches and that ultimately gives rise to the jaws and the skeleton of the gill -

Functional Structure of the Skull and Fractures of the Skull Thickened and Thinner Parts of the Skull
Functional structure of the skull and Fractures of the skull Thickened and thinner parts of the skull = important base for understanding of the functional structure of the skull → - the transmission of masticatory forces - fracture predilection Thickned parts: . sagittal line . ventral lateral line . dorsal lateral line Thinner parts: . articular fossa . cribriform plate . foramines, canals and fissures . anterior, medial and posterior cranial fossa Thickned parts: . tuber parietalis . mastoid process . protuberantia occipitalis ext. et int. linea temporalis . margin of sulcus sinus: - sagitalis sup. - transversus Functional structure of the skull Facial buttresses system . Of thin segments of bone encased and supported by a more rigid framework of "buttresses" . The midface is anchored to the cranium through this framework . Is formed by strong frontal, maxillary, zygomatic and sphenoid bones and their attachments to one another Tuber maxillae Vertical buttress Sinus maxillae Orbita . nasomaxillary Nasal cavity . zygomaticomaxillary . pterygomaxillary Horizontal buttress . glabella . orbital rims . zygomatic processes . maxillary palate . The buttress system absorbs and transmits forces applied to the facial skeleton . Masticatory forces are transmitted to the skull base primarily through the vertical buttresses, which are joined and additionally supported by the horizontal buttresses . When external forces are applied, these components prevent disruption of the facial skeleton until a critical level is reached and then fractures occur Stress that occurs from mastication or trauma is transferred from the inferior of the mandible via various trajectory lines → to the condyles glenoid fossa → temporal bone The main alveolar stress concentration were located interradicularly and interproximally Fractures of the skull I. Neurocranial fractures II. Craniofacial fractures I. Neurocranial fracture . A break in the skull bone are generally occurs as a result of a direct impact . -

Metastatic Skull Tumours: Diagnosis and Management Mitsuya K, Nakasu Y European Association of Neurooncology Magazine 2014; 4 (2) 71-74
Volume 4 (2014) // Issue 2 // e-ISSN 2224-3453 European Association of NeuroOncology Magazine Neurology · Neurosurgery · Medical Oncology · Radiotherapy · Paediatric Neuro- oncology · Neuropathology · Neuroradiology · Neuroimaging · Nursing · Patient Issues Metastatic Skull Tumours: Diagnosis and Management Mitsuya K, Nakasu Y European Association of NeuroOncology Magazine 2014; 4 (2) 71-74 Homepage www.kup.at/ journals/eano/index.html Online Database Featuring Author, Key Word and Full-Text Search Member of the THE EUROPEAN ASSOCIATION OF NEUROONCOLOGY Indexed in EMBASE Krause & Pachernegg GmbH . VERLAG für MEDIZIN und WIRTSCHAFT . A-3003 Gablitz, Austria Metastatic Skull Tumours Metastatic Skull Tumours: Diagnosis and Management Koichi Mitsuya, Yoko Nakasu Abstract: Metastases of the skull are classifi ed Magnetic resonance imaging is the primary diag- skull metastasis. Surgery is indicated in selected into 2 anatomical groups, presenting distinct clin- nostic tool. Skull metastasis is a focal lesion with patients with good performance status who need ical features. One is calvarial metastasis, which a low-intensity signal on T1-weighted images. En- immediate decompression, cosmetic recovery, or is usually asymptomatic but may cause dural in- hanced T1-weighted images with fat-suppression histological diagnosis. Eur Assoc NeuroOncol vasion, dural sinus occlusion, or cosmetic prob- show tumour, dural infi ltration, and cranial nerve Mag 2014; 4 (2): 71–4. lems. The other is skull-base metastasis, which involvements. Irradiation is the effective and fi rst- presents with cranial-nerve involvement leading line therapy for most skull metastases. Chemo- to devastating symptoms. A high index of suspi- therapy or hormonal therapy is applied depending cion based on new-onset cranial nerve defi cits on tumour sensitivity.