Annual Review 2003
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Review 2005
GS2184_Review_A\W2.qxd 7/3/06 4:58 pm Page fc1 Annual Review 2005 human being Do more, feel better, live longer GS2184_Review_A\W2.qxd 9/3/06 1:28 pm Page ifc2 01 An interview with Sir Christopher Gent, Chairman, and JP Garnier, Chief Executive Officer 05 Tachi Yamada, Chairman, Research & Development, Pharmaceuticals 06 Jean Stéphenne, President and General Manager, GSK Biologicals 08 John Clarke, President, Consumer Healthcare 11 David Stout, President, Pharmaceutical Operations 14 Performance highlights 15 Business operating review 18 The Board 19 The Corporate Executive Team 20 Summary remuneration report 23 Corporate governance 24 Responsibility statements 25 Summary financial statements 26 Summary information under US GAAP 27 Shareholder information 29 Chairman and CEO’s closing letter JP Garnier (left) and Sir Christopher Gent (right) GS2184_Review_A\W2.qxd 7/3/06 5:01 pm Page 01 “Discovering important medicines, eradicating diseases, improving the quality of people’s lives and making medicines available to a greater number of people. This is what we do – and what we do matters to people.” JP Garnier, Chief Executive Officer An interview with Sir Christopher Gent, Chairman and JP Garnier, Chief Executive Officer 2005: a year of success and progress “Thanks to the efforts of our employees around the company’s pipeline is one of the largest and most world, 2005 was a very successful year for GSK,” says promising in the industry, with 149 projects in clinical JP Garnier, Chief Executive Officer. “Not only was it development (as at the end of February 2006), our best year ever from a financial standpoint, we also including 95 new chemical entities (NCEs), 29 product made substantial progress with our pipeline of line extensions (PLEs) and 25 vaccines. -

2005 GSK Corporate Responsibility Report
2005 GSK Corporate Responsibility Report Introduction 1 Introduction Page Our contribution to society 1 About this report 1 CEO and Chairman’s letter 3 Managing corporate responsibility 4 Why CR is important to GSK 4 CR governance 4 Our CR principles 6 Stakeholder engagement 7 Stakeholder feedback 7 How we are responding 9 Government and external affairs 10 Membership of trade associations 10 US lobbying expenditures 10 Political donations 10 Our position on key issues 11 Patient advocacy 11 2 Access to medicine 3 Research 4 Ethical conduct 5 Employment practices 6 Human rights 7 Environment 8 Community investment 9 Data summary 1 1 GSK CORPORATE RESPONSIBILITY REPORT 2005 Introduction Welcome to GSK’s Corporate Responsibility Report 2005. This report explains our approach to the wide range of social, ethical and environmental issues associated with our business and reports our performance in 2005. The full web-based report is available on www.gsk.com GSK is a research-based pharmaceutical company, with operations in 119 countries. We make prescription medicines, vaccines, over-the-counter medicines, and consumer healthcare products. Our business accounts for 6.3% of the world’s pharmaceutical market. We have strong positions in several therapeutic areas including anti-infectives, asthma, cancer, cardiovascular, depression, diabetes, HIV/AIDS and urology. For an overview of our business, see our Annual Report. OUR CONTRIBUTION TO SOCIETY This report explains our approach to the significant In the last 80 years, medicines and vaccines have corporate responsibility issues for our business, including: transformed millions of lives. They have helped to increase • Access to medicines – how we make our medicines life expectancy and lowered death rates from conditions accessible to poor patients in developed and developing such as heart disease, stroke and cancer. -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -
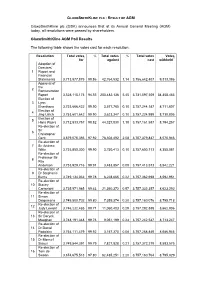
Voting/Poll Results
GLAXOSMITHKLINE PLC - RESULT OF AGM GlaxoSmithKline plc (GSK) announces that at its Annual General Meeting (AGM) today, all resolutions were passed by shareholders. GlaxoSmithKline AGM Poll Results The following table shows the votes cast for each resolution: Resolution Total votes % Total votes % Total votes Votes for* against cast withheld** Adoption of Directors’ 1 Report and Financial Statements 3,713,877,875 98.86 42,764,532 1.14 3,756,642,407 9,313,386 Approval of the 2 Remuneration Report 3,528,115,173 94.55 203,482,136 5.45 3,731,597,309 34,358,483 Election of 3 Lynn Elsenhans 3,753,666,422 99.90 3,577,765 0.10 3,757,244,187 8,711,607 Election of 4 Jing Ulrich 3,753,601,642 99.90 3,623,347 0.10 3,757,224,989 8,730,805 Election of 5 Hans Wijers 3,712,833,757 98.82 44,327,830 1.18 3,757,161,587 8,794,257 Re-election of Sir 6 Christopher Gent 3,679,075,355 97.92 78,304,492 2.08 3,757,379,847 8,575,946 Re-election of 7 Sir Andrew Witty 3,753,850,300 99.90 3,750,413 0.10 3,757,600,713 8,355,081 Re-election of Professor Sir 8 Roy Anderson 3,753,929,716 99.91 3,483,857 0.09 3,757,413,573 8,542,221 Re-election of 9 Dr Stephanie Burns 3,749,134,033 99.78 8,228,665 0.22 3,757,362,698 8,592,951 Re-election of 10 Stacey Cartwright 3,735,971,985 99.43 21,360,372 0.57 3,757,332,357 8,623,292 Re-election of 11 Simon Dingemans 3,749,800,702 99.80 7,359,374 0.20 3,757,160,076 8,795,718 Re-election of 12 Judy Lewent 3,746,232,485 99.71 11,060,403 0.29 3,757,292,888 8,662,906 Re-election of 13 Sir Deryck Maughan 3,748,191,348 99.76 9,051,199 0.24 -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Corporate Responsibility Report 2008
Corporate Responsibility Report 2008 Strength through responsibility Summary Reed Elsevier Corporate Responsibility Report 2008 Chief Executive’s introduction Governance People Our true performance as a company must take account of both our non-financial and financial results. They are intrinsically linked – if we want to be a profitable and successful company, we must be an ethical one. We are committed to being both. To that end, we have been building on our expertise and engaging with stakeholders to find ways to innovate and improve. Health and safety In developing online solutions to the needs of our customers in areas like health, science and technology, law, environment, and business, we benefit society. For example, Procedures Consult gives doctors a way of maintaining their skills and knowledge through web-based simulation of essential medical techniques; TotalPatent is a single source for global patents, a primary driver in research and development; XpertHR helps practitioners advance good practice in people management; energylocate aggregates our energy offerings into a one-stop community, using tools like social networking and video to advance knowledge. Customers Innovation is also helping advance our internal corporate responsibility: > Increased online training in our Code of Ethics and Business Conduct has led to more of our people understanding what it means to do the right thing > A new global jobs board illuminates our value, Boundarylessness, by allowing employees to search for new positions across locations, functions, -

22 November 2012 • No 5007 • Vol 143 Gazette
ThursdaY 22 november 2012 • no 5007 • vol 143 Gazette Council and Main Notices 179 Advertisements 183 Committees 176 Consultative notices: Council of the University: Consolidation of small trust funds Notifications of Vacancies 186 Approval of nomination of external member of Council General notices: University of Oxford Gazette publication arrangements Council of the University: Colleges, Halls and Societies Changes in Regulations: Appointments: External Vacancies (a) Committees reporting directly to University Administration and Council or one of its main Services Supplement included with this issue: committees (1) to no 5006: Topic for discussion: (b) Regulations for the Student Visiting Professorships: the libraries and their future 159–174 Fitness to Study Panel Medical Sciences (c) Delegacy for Military Instruction (d) Personnel Committee Electoral Boards: (e) Research Committee Professorship of Economics (f) Socially Responsible Investment Newton Abraham Visiting Review Committee Professorship in the Medical, (g) Income grant from the College Biological and Chemical Sciences Contributions Fund Musical and other Events: General Purposes Committee of Council: lincoln Changes in Regulations: Exhibitions: Saïd Business School and Business Magdalen Advisory Council Council of the University: Lectures 181 Register of Congregation Examinations and Boards 181 Congregation 178 Examinations for the Degree of Doctor of Congregation 26 November: Philosophy Degree by Resolution Examinations for the Degree of Master of Congregation 27 november: -

Vote Summary
Vote Summary FENNER PLC, HESSLE YORKSHIRE Security G33656102 Meeting Type Annual General Meeting Ticker Symbol Meeting Date 14-Jan-2015 ISIN GB0003345054 Agenda 705747369 - Management Record Date Holding Recon Date 12-Jan-2015 City / Country LONDON / United Vote Deadline Date 08-Jan-2015 Kingdom SEDOL(s) 0334505 - B3BH6Q8 Quick Code Proposal Proposed Vote For/Against by Manageme nt Item 1 TO RECEIVE THE REPORTS OF THE DIRECTORS Management For For 2 TO APPROVE THE BOARD REMUNERATION POLICY Management For For 3 TO APPROVE THE BOARD ANNUAL REMUNERATION Management For For 4 TO DECLARE A DIVIDEND Management For For 5 TO RE-ELECT MARK ABRAHAMS Management For For 6 TO RE-ELECT NICHOLAS HOBSON Management For For 7 TO RE-ELECT RICHARD PERRY Management For For 8 TO RE-ELECT VANDA MURRAY Management For For 9 TO RE-ELECT JOHN SHELDRICK Management For For 10 TO RE-ELECT ALAN WOOD Management For For 11 TO RE-APPOINT THE AUDITORS Management For For 12 TO AUTHORISE THE DIRECTORS TO DETERMINE Management For For 13 TO APPROVE THE FENNER PERFORMANCE SHARE Management For For 14 AUTHORITY TO ALLOT SHARES Management For For 15 POWER TO ALLOT SHARES FOR CASH AND Management For For 16 AUTHORITY TO BUY BACK SHARES Management For For 17 TO ALLOW THE COMPANY TO HOLD GENERAL Management For For IMPERIAL BRANDS PLC, BRISTOL Security G4721W102 Meeting Type Annual General Meeting Ticker Symbol Meeting Date 28-Jan-2015 ISIN GB0004544929 Agenda 705751356 - Management Record Date Holding Recon Date 26-Jan-2015 City / Country BRISTOL / United Vote Deadline Date 22-Jan-2015 -

Notice of Annual General Meeting
This document is important and requires your immediate attention If you are in any doubt as to what action you should take, you should consult immediately with your stockbroker, bank manager, solicitor, accountant or other independent professional adviser authorised under the Financial Services and Markets Act 2000. If you have sold or otherwise transferred all your shares in Reed Elsevier PLC, please send this Notice of Annual General Meeting and accompanying documents to the stockbroker, or other agent through whom the sale or transfer was effected, for transmission to the purchaser or transferee. A Form of Proxy for the Annual General Meeting is enclosed and should be completed and returned so as to reach the company’s Registrar by no later than 11.00 am on Monday 21 April 2008. Completion and return of the Form of Proxy will not prevent you from attending and voting at the meeting in person, should you wish. Alternatively, you can register your proxy vote electronically, either by means of a website provided by the company’s Registrar at www.sharevote.co.uk, or by using the service provided by Euroclear UK & Ireland Limited. Further details are given in the notes to the attached Notice of Annual General Meeting. Reed Elsevier PLC Notice of Annual General Meeting to be held at the Millennium Hotel, Grosvenor Square, London, W1K 2HP on Wednesday, 23 April 2008 at 11.00 am 2 Notice of Annual General Meeting To the holders of Reed Elsevier PLC ordinary shares Dear Shareholder, Annual General Meeting 2008 Introduction I am pleased to invite you to the Annual General Meeting of the company for 2008, which will be held on Wednesday, 23 April 2008 at 11.00 am in the Ballroom at the Millennium Hotel, Grosvenor Square, London, W1K 2HP. -

FORM N-PX Annual Report of Proxy Voting Record of Registered Management Investment Companies Filed on Form N-PX
SECURITIES AND EXCHANGE COMMISSION FORM N-PX Annual report of proxy voting record of registered management investment companies filed on Form N-PX Filing Date: 2021-08-13 | Period of Report: 2021-06-30 SEC Accession No. 0000894189-21-005339 (HTML Version on secdatabase.com) FILER HOTCHKIS & WILEY FUNDS /DE/ Mailing Address Business Address 601 SOUTH FIGUEROA 601 SOUTH FIGUEROA CIK:1145022| IRS No.: 000000000 | State of Incorp.:DE | Fiscal Year End: 0630 STREET STREET Type: N-PX | Act: 40 | File No.: 811-10487 | Film No.: 211171831 39TH FLOOR 39TH FLOOR LOS ANGELES CA LOS ANGELES CA 90017-5704 90017-5704 2134301000 Copyright © 2021 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM N-PX ANNUAL REPORT OF PROXY VOTING RECORD OF REGISTERED MANAGEMENT INVESTMENT COMPANY Investment Company Act file number 811-10487 Hotchkis and Wiley Funds -------------------------------------------------------------------------------- (Exact name of registrant as specified in charter) 601 S. Figueroa Street, 39th Floor, Los Angeles, CA 90017 -------------------------------------------------------------------------------- (Address of principal executive offices) (Zip code) Anna Marie Lopez 601 S. Figueroa Street, 39th Floor Los Angeles, CA 90017 -------------------------------------------------------------------------------- (Name and address of agent for service) Registrant's telephone number, including area code: 1-213-430-1000 Date -

Annual Report 2008 Find out More About GSK Online…
Do more, feel better, live longer Grow Deliver Simplify Annual Report 2008 Find out more about GSK online… www.gsk.com Website GlaxoSmithKline’s website www.gsk.com gives additional information on the Group. Information made available on the website does not constitute part of this Annual Report. Notice regarding limitations on Director liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Report of the Directors contained on pages 12 to 98. Under English law the Directors would be liable to the company (but not to any third party) if the Report of the Directors contains errors as a result of recklessness or knowing misstatement or dishonest concealment of a material fact, but would not otherwise be liable. Report of the Directors Pages 12 to 98 inclusive consist of a Report of the Directors that has been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with that report shall be subject to the limitations and restrictions provided by such law. Cautionary statement regarding forward-looking statements The Group’s reports filed with or furnished to the US Securities and Exchange Commission (SEC), including this document and written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. A shareholder can identify these statements by the fact that they do not relate strictly to historical or current facts. -

Printmgr File
Dear Fellow Shareholders, Hasbro will host the 2017 Annual Meeting of Shareholders on Thursday, May 18 at the Company’s headquarters in Pawtucket, Rhode Island. The attached Notice of Annual Meeting of Shareholders and Proxy Statement provides information regarding the business we will conduct at the Meeting and other important matters regarding our Company. On behalf of Hasbro’s Board of Directors, we encourage you to vote your shares and invite you to join the meeting. Over the past ten years, Hasbro has redefined itself as a global organization Creating the World’s Best Play Experiences. It has invested to build the best possible team to execute consumer insight and story-led brands across consumer touch points, including toys and games, digital gaming, entertainment and consumer products. This strategic roadmap is known as Hasbro’s Brand Blueprint. In 2016, strong execution of the Brand Blueprint delivered another record year. 13% revenue growth drove Hasbro’s first $5 billion revenue year along with a 14% increase in operating profit and a 22% increase in diluted earnings per share. Consistent with Hasbro’s long-standing capital priorities, the Company invested back into the business to drive long-term profitable growth and shareholder value creation. In 2016, this included investing in brands, innovation, new capabilities around the Brand Blueprint, such as the acquisition of Boulder Media, and in systems infrastructure. The Company then returned excess cash to shareholders. Hasbro generated $775 million in operating cash flow last year and returned approximately $400 million to shareholders. This included $249 million in dividends and $151 million in share repurchases.