Abundance, Biomass and Energy Use of Native and Alien Breeding Birds in Britain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scottish Birds 22: 9-19
Scottish Birds THE JOURNAL OF THE SOC Vol 22 No 1 June 2001 Roof and ground nesting Eurasian Oystercatchers in Aberdeen The contrasting status of Ring Ouzels in 2 areas of upper Deeside The distribution of Crested Tits in Scotland during the 1990s Western Capercaillie captures in snares Amendments to the Scottish List Scottish List: species and subspecies Breeding biology of Ring Ouzels in Glen Esk Scottish Birds The Journal of the Scottish Ornithologists' Club Editor: Dr S da Prato Assisted by: Dr I Bainbridge, Professor D Jenkins, Dr M Marquiss, Dr J B Nelson, and R Swann Business Editor: The Secretary sac, 21 Regent Terrace Edinburgh EH7 5BT (tel 0131-5566042, fax 0131 5589947, email [email protected]). Scottish Birds, the official journal of the Scottish Ornithologists' Club, publishes original material relating to ornithology in Scotland. Papers and notes should be sent to The Editor, Scottish Birds, 21 Regent Terrace, Edinburgh EH7 SBT. Two issues of Scottish Birds are published each year, in June and in December. Scottish Birds is issued free to members of the Scottish Ornithologists' Club, who also receive the quarterly newsletter Scottish Bird News, the annual Scottish Bird Report and the annual Raplor round up. These are available to Institutions at a subscription rate (1997) of £36. The Scottish Ornithologists' Club was formed in 1936 to encourage all aspects of ornithology in Scotland. It has local branches which meet in Aberdeen, Ayr, the Borders, Dumfries, Dundee, Edinburgh, Glasgow, Inverness, New Galloway, Orkney, St Andrews, Stirling, Stranraer and Thurso, each with its own programme of field meetings and winter lectures. -

Eds. 1965. Princeton, New Jersey, Princeton Univ. Press. Pp. X Q- 922
REVIEWS EDITED BY KENNETH C. PARKES The Quaternary of the United States.--H. E. Wright, Jr., and David G. Frey, eds.1965. Princeton,New Jersey,Princeton Univ. Press.Pp. x q- 922, illus., 11 in. $25.00.--Knowledgeis accumulatingvery rapidly in the generalarea of Pleistocene q- Recent (= Quaternary) biogeographyand evolutionary history. This exponential growth resultsfrom the rapid developmentof improvedmethods of dating orga.nic matter (radiocarbon,potassium argon, etc.), in pollen core analysis,in glacial and extra-glacial stratigraphy, in oceanography,and in other areas, as well as from acceleratedprosecution of classicalpaleontological and biogeographicanalyses. Con- sequently,review papers and symposiagrow out of date almost as rapidly as they are published. Resulting from the seventh and latest Congressof the International Association for QuaternaryResearch (INQUA), at Boulder,Colorado, in 1965,the presentmassive volume supersedesall of its predecessorsfor the area in question (which, despite the title, variously relatesto most of North America). Each review paper is complete in itself with its own bibliography; there are terminal indices to all. Emphasis is divided among Parts I-IV (Geology; Biogeography;Archaeology; Miscellaneous). While general background is always useful, Part II is obviously of the greatest immediate interest to ornithologists. It is divided among"Phytogeography and Palynology" (pollen analysis), "Zoogeog- raphy and Evolution," and a summarizing"Pleistocene Nonmarine Environments" (by E. S. Deevey, Jr.). Under the second sub-heading are chapters on mammals (by C. W. Hibbard and four other active authors), birds ("Avian Speciation in the Quaternary"; pp. 529-542, by Robert K. Selander), amphibians (by W. Frank Blair), reptiles (by Walter Auffenberg and William W. -

Changes to Category C of the British List†
Ibis (2005), 147, 803–820 Blackwell Publishing, Ltd. Changes to Category C of the British List† STEVE P. DUDLEY* British Ornithologists’ Union, Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK In its maintenance of the British List, the British Ornithologists’ Union Records Committee (BOURC) is responsible for assigning species to categories to indicate their status on the List. In 1995, the British Ornithologists’ Union (BOU) and the Joint Nature Conservation Committee (JNCC) held a conference on naturalized and introduced birds in Britain (Holmes & Simons 1996). This led to a review of the process of establishment of such species and the terms that best describe their status (Holmes & Stroud 1995) as well as a major review of the categorization of species on the British List (Holmes et al. 1998). The BOURC continues to review the occurrence and establishment of birds of captive origin in Britain. This paper sum- marizes the status of naturalized and introduced birds in Britain and announces changes to the categorization of many on the British List or its associated appendices (Categories D and E): Mute Swan Cygnus olor Categories AC change to AC2 Black Swan Cygnus atratus Category E* – no change Greylag Goose Anser anser Categories ACE* change to AC2C4E* Snow Goose Anser caerulescens Categories AE* change to AC2E* Greater Canada Goose Branta canadensis Categories ACE* change to C2E* Barnacle Goose Branta leucopsis Categories AE* change to AC2E* Egyptian Goose Alopochen aegyptiacus Categories CE* change -

Golden Pheasant Chrysolophus Pictus
Young Golden Pheasant Chrysolophus pictus What is the history of my relationship to man? The Golden Pheasant is commonly found in zoos and aviaries, but often as impure specimens that have the similar Lady Amherst's Pheasant in their lineage. Habitat / Climate Where am I from? dark young conifer forests The Golden Pheasant or "Chinese Pheasant",is a parrot like Map with sparse undergrowth gamebird of the order Galliformes. It is native to forests in mountainous areas of western China but feral populations have been established in the United Kingdom and elsewhere. Other family members: Caspian snowcock Who are my relatives? Gray partridge Silver Pheasant, Little chachalaca, Yellow- knobbed curassow and Western capercaillie the Turkey Breeding Potential How am I born? We lay 8-12 eggs at a time and will then incubate these for around 22-23 days. Clutch size 8 to 12 eggs When we hatch we are able to walk and look for food with in hours. By a few weeks we will loose our down and have our feathers in. How long does it take me to grow up and how long do I live Once we have gotten our feathers we will keep getting bigger. By 3 months we will be full Breeding Season grown, we might add a few pounds after that but wont get larger. We can live up to 6 years. J F M A M J J A S O N D A E A P A U U U E C O E N B R R Y N L G P T V C What kind of family life do I have? We are extremely territorial. -

( 133 ) on the Yellow Wagtails, and Their
( 133 ) ON THE YELLOW WAGTAILS, AND THEIR POSITION IN THE BRITISH AVIFAUNA. BY N. F. TICEHURST, F.R.C.S., M.B.O.U. IN 1832 the late John Gould pointed out that the British Yellow Wagtail was a different species from that inhabiting the nearest parts of the continent and at the same time clearly showed that, while the continental bird was of rare occurrence in this country, our common species was almost equally rare on the continent.* The common Yellow Wagtail (Motacilla rail, Bonaparte) is a regular summer migrant to the British islands, which form its head-quarters throughout the breeding season. It arrives on our south coast during the last ten days of March, throughout April and during the first week of May, the males generally appearing a full fortnight before the females. Jts breeding range extends from the southern counties of England as far west as Somerset, northward to Inverness and Aberdeen, throughout which area it is generally distributed in suitable localities. In Devon and Cornwall it occurs chiefly as a spring and autumn migrant, though in the former county it breeds in limited numbers. In Wales it is local as a breeding species, while to the north of Scotland it can only be regarded as a rare visitor. It is said to have occurred in the Shetlands, and an adult male was obtained on Fair Isle, 8th May, 1906, and in Ireland it is extremely local, breeding in one or two localities only. In most parts of the continent it occurs only as a straggler during the periods of migration, but in France it is found in summer as a breeding species to the west of " Proc. -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Assessment of Hematological Indices of Indian Peafowl (Pavo Cristatus) Kept at Wildlife Breeding Centre, Gatwala, Faisalabad, Pakistan
Journal of Zoological Research Volume 4, Issue 1, 2020, PP 29-33 ISSN 2637-5575 Assessment of Hematological Indices of Indian Peafowl (Pavo Cristatus) Kept at Wildlife Breeding Centre, Gatwala, Faisalabad, Pakistan Misbah Sarwar1* Zahid Ali1, and Muhammad Bilal Chaudhary2 1Punjab Wildlife Research Centre, Gatwala, Faisalabad Department of Wildlife & Parks, Punjab Pakistan 2Department of Zoology, GC University, Faisalabad, Pakistan *Corresponding Author: Misbah Sarwar, Punjab Wildlife Research Centre, Gatwala, Faisalabad Department of Wildlife & Parks, Punjab Pakistan. Email: [email protected] ABSTRACT Indian peafowl (Pavo cristatus) or blue peafowl has been maintained in captivity since long where due to selective breeding, several color mutations/varieties have appeared of which white peafowl, black-shouldered peafowl and pied peafowl are common. Since, hematological analysis is crucial for clinical diagnosis of wild and captive avifauna, so we collected blood samples from healthy male blue peafowl, white peafowl and black- shouldered peafowl kept at Wildlife Breeding Centre, Gatwala, Faisalabad and compared erythrocyte and leucocyte indices among them. Our results indicated that blood physiological values for MO (%), Hgb, HCT, MCH and MCHC were significantly different (P<0.05) between blue peafowl and white peafowl whereas MCV and RDW were significantly different (P<0.05) between blue peafowl and black-shouldered peafowl. The comparison of hematological parameters between white peafowl and black-shouldered peafowl showed that GR(%), RBC, HCT, MCV and MCHC differ significantly (P<0.05) between the two varieties. Our results support the studies indicating high quality color patterns reflect increased resistance and immunity to pathogens. Keywords: Indian peafowl, Color Mutations, hematology. INTRODUCTION 2005; Takahashi and Hasegawa, 2008; Harikrishnan et al., 2010, Naseer et al., 2017). -

Merlewood RESEARCH and DEVELOPMENT PAPER No 84 A
ISSN 0308-3675 MeRLEWOOD RESEARCH AND DEVELOPMENT PAPER No 84 A BIBLIOGRAPHICALLY-ANNOTATED CHECKLIST OF THE BIRDS OF SAETLAND by NOELLE HAMILTON Institute of Terrestrial Ecology Merlewood Research Station Grange-over-Sands Cumbria England LA11 6JU November 1981 TABLE OF CONTENTS INTRODUCTION 1-4 ACKNOWLELZEMENTS 4 MAIN TEXT Introductory Note 5 Generic Index (Voous order) 7-8 Entries 9-102 BOOK SUPPUNT Preface 103 Book List 105-125 APPENDIX I : CHECKLIST OF THE BIRDS OF SBETLAND Introductar y Note 127-128 Key Works : Books ; Periodicals 129-130 CHECKLI5T 131-142 List of Genera (Alphabetical) 143 APPENDICES 11 - X : 'STATUS' IJSTS Introductory Note 145 11 BREEDING .SPECIES ; mainly residem 146 111 BREEDING SPECIES ; regular migrants 147 N NON-BREEDING SPECIES ; regular migrants 148 V NON=BREEDING SPECIES ; nationally rare migrants 149-150 VI NON-BREEDING SPECIES ; locally rare migrants 151 VII NON-BREEDING SPECIES : rare migrants - ' escapes ' ? 152 VIII SPECIES RECORDED IN ERROR 153 I IX INTRDWCTIONS 154 X EXTINCTIONS 155 T1LBLE 157 INTRODUCTION In 1973, in view of the impending massive impact which the then developing North Sea oil industry was likely to have on the natural environment of the Northern Isles, the Institute of Terrestrial Ecology was commissioned iy the Nature Conservancy Council to carry out an ecological survey of Shetland. In order to be able to predict the long-term effects of the exploitation of this important and valuable natural resource it was essential to amass as much infarmation as passible about the biota of the area, hence the need far an intensive field survey. Subsequently, a comprehensive report incorporating the results of the Survey was produced in 21 parts; this dealt with all aspects of the research done in the year of the Survey and presented, also, the outline of a monitoring programme by the use of which it was hoped that any threat to the biota might be detected in time to enable remedial action to be initiated. -

CHAPTER 5 PRODUCTS of ANIMAL ORIGIN, NOT ELSEWHERE SPECIFIED OR INCLUDED I 5-1 Notes: 1
)&f1y3X CHAPTER 5 PRODUCTS OF ANIMAL ORIGIN, NOT ELSEWHERE SPECIFIED OR INCLUDED I 5-1 Notes: 1. This chapter does not cover: (a) Edible products (other than guts, bladders and stomachs of animals, whole and pieces thereof, and animal blood, liquid or dried); (b) Hides or skins (including furskins) other than goods of heading 0505 and parings and simlar waste of raw hides or skins of heading 0511 (chapter 41 or 43); (c) Animal textile materials, other than horsehair and horsehair waste (section XI); or (d) Prepared knots or tufts for broom or brush making (heading 9603). 2. For the purposes of heading 0501, the sorting of hair by length (provided the root ends and tip ends, respectively, are not arranged together) shall be deemed not to constitute working. 3. Throughout the tariff schedule, elephant, walrus, narwhal and wild boar tusks, rhinoceros horns and the teeth of all animals are regarded as "ivory." 4. Throughout the tariff schedule, the expression "horsehair" means hair of the manes or tails of equine or bovine animals. Additional U.S. Note 1. (a) Except as provided in paragraphs (b) and (c) of this note, the importation of the feathers or skin of any bird is hereby prohibited. Such prohibition shall apply to the feathers or skin of any bird: (i) Whether raw or processed; (ii) Whether the whole plumage or skin or any part of either; (iii) Whether or not attached to a whole bird or any part thereof; and (iv) Whether or not forming part of another article. (b) Paragraph (a) shall not apply: (i) In respect of any of the following birds (other than any such bird which, whether or not raised in captivity, is a wild bird): chickens (including hens and roosters), turkeys, guineas, geese, ducks, pigeons, ostriches, rheas, English ring-necked pheasants and pea fowl; (ii) To any importation for scientific or educational purposes; (iii) To the importation of fully manufactured artificial flies used for fishing; (iv) To the importation of birds which are classifiable under subheading 9804.00.55; and (v) To the importation of live birds. -

Print 02/02 February
The non-breeding status of the Little Egret in Britain A. J. Musgrove Mike Langman ABSTRACT The Little Egret Egretta garzetta, formerly a rare vagrant in Britain, has undergone a remarkable range expansion following a large influx in 1989, and at the end of 1990 it was removed from the list of species considered by the British Birds Rarities Committee. Single-site counts in excess of 100 were made in autumn 1995, with the first site count of more than 200 in autumn 1998.The Little Egret was confirmed as a new British breeding bird in 1996, and by 1999 at least 30 breeding pairs were recorded at a total of nine sites. This paper documents the change in the species’ non-breeding status in Britain. Using data from the Wetland Bird Survey, with supplementary counts from roost surveys and county bird reports, it is estimated that more than 1,650 Little Egrets were present in September 1999, over 40% of these between Swanage Bay, Dorset, and Pagham Harbour,West Sussex. A further increase in the British population is expected.The increase in Britain has been mirrored in adjacent areas of northwest Europe. 62 © British Birds 95 • February 2002 • 62-80 Non-breeding status of Little Egret ormerly a rare vagrant in Britain, the Palearctic, very few were reported in the nine- Little Egret Egretta garzetta has undergone teenth century and in the first half of the twen- Fa remarkable range expansion in recent tieth century. In fact, certain well-watched years. Following a large influx into Britain in counties did not record the species until com- 1989, and continued high numbers during paratively recently, with first county records for 1990, the British Birds Rarities Committee took Sussex in 1952 (James 1996), Norfolk in 1952 the decision to remove the Little Egret from the (Taylor et al. -

Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa
Journal of Heredity 2003:94(6):472–489 Ó 2003 The American Genetic Association DOI: 10.1093/jhered/esg092 Phylogenetic Relationships of the Phasianidae Reveals Possible Non-Pheasant Taxa K. L. BUSH AND C. STROBECK From the Department of Biological Sciences, University of Alberta–Edmonton, Edmonton, Alberta T6G 2E9, Canada. Address correspondence to Krista Bush at the address above, or e-mail: [email protected]. Abstract The phylogenetic relationships of 21 pheasant and 6 non-pheasant species were determined using nucleotide sequences from the mitochondrial cytochrome b gene. Maximum parsimony and maximum likelihood analysis were used to try to resolve the phylogenetic relationships within Phasianidae. Both the degree of resolution and strength of support are improved over previous studies due to the testing of a number of species from multiple pheasant genera, but several major ambiguities persist. Polyplectron bicalcaratum (grey peacock pheasant) is shown not to be a pheasant. Alternatively, it appears ancestral to either the partridges or peafowl. Pucrasia macrolopha macrolopha (koklass) and Gallus gallus (red jungle fowl) both emerge as non-pheasant genera. Monophyly of the pheasant group is challenged if Pucrasia macrolopha macrolopha and Gallus gallus are considered to be pheasants. The placement of Catreus wallichii (cheer) within the pheasants also remains undetermined, as does the cause for the great sequence divergence in Chrysolophus pictus obscurus (black-throated golden). These results suggest that alterations in taxonomic -
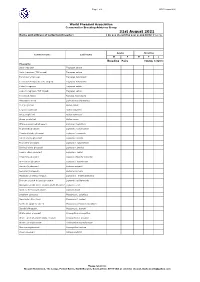
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor