Trajectories of Cholinergic Pathways Within the Cerebral Hemispheres of the Human Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synaptic Organization of Claustral and Geniculate Afferents to the Visual Cortex of the Cat
The Journal of Neuroscience December 1986, 6(12): 3564-3575 Synaptic Organization of Claustral and Geniculate Afferents to the Visual Cortex of the Cat Simon LeVay Robert Bosch Vision Research Center, Salk Institute for Biological Studies, San Diego, California 92138 Claustral and geniculate afferents to area 17 were labeled by sponses of some cortical neurons, previously called “hypercom- anterograde axonal transport of peroxidase-conjugated wheat- plex cells” by Hubel and Wiesel (1965), are suppressed as the germ agglutinin, and examined in the electron microscope. A length of the stimulating slit of light is extended beyond some peroxidase reaction protocol that led to labeling in the form of optimal value. Interestingly, neurons in the visual claustrum minute holes in the EM sections was used. Both types of affer- behave in a complementary fashion: Their responses to an ori- ents formed type 1 (presumed excitatory) synapses exclusively. ented stimulus increase monotonically with stimulus length, In agreement with previous reports the great majority of genic- sometimes showing length summation up to 40” or more-a ulate afferents to layers 4 and 6 contacted dendritic spines. The sizable portion of the animal’s entire field of view (Sherk and claustral afferents to layers 1 and 6 also predominantly con- LeVay, 198 1). tacted spines. In layer 4, however, claustral afferents contacted These observations suggest that claustral neurons contribute spines and dendritic shafts about equally. The results suggest a to end-stopping by exerting a length-dependent inhibition on substantial Claus&al input to smooth-dendrite cells in layer 4, some neurons in the visual cortex. -

The Effect of Nucleus Basalis Magnocellularis Deep Brain
Lee et al. BMC Neurology (2016) 16:6 DOI 10.1186/s12883-016-0529-z RESEARCH ARTICLE Open Access The effect of nucleus basalis magnocellularis deep brain stimulation on memory function in a rat model of dementia Ji Eun Lee1†, Da Un Jeong1†, Jihyeon Lee1, Won Seok Chang2 and Jin Woo Chang1,2* Abstract Background: Deep brain stimulation has recently been considered a potential therapy in improving memory function. It has been shown that a change of neurotransmitters has an effect on memory function. However, much about the exact underlying neural mechanism is not yet completely understood. We therefore examined changes in neurotransmitter systems and spatial memory caused by stimulation of nucleus basalis magnocellularis in a rat model of dementia. Methods: We divided rats into four groups: Normal, Lesion, Implantation, and Stimulation. We used 192 IgG-saporin for degeneration of basal forebrain cholinergic neuron related with learning and memory and it was injected into all rats except for the normal group. An electrode was ipsilaterally inserted in the nucleus basalis magnocellularis of all rats of the implantation and stimulation group, and the stimulation group received the electrical stimulation. Features were verified by the Morris water maze, immunochemistry and western blotting. Results: AllgroupsshowedsimilarperformancesduringMorriswater maze training. During the probe trial, performance of the lesion and implantation group decreased. However, the stimulation group showed an equivalent performance to the normal group. In the lesion and implantation group, expression of glutamate acid decarboxylase65&67 decreased in the medial prefrontal cortex and expression of glutamate transporters increased in the medial prefrontal cortex and hippocampus. However, expression of the stimulation group showed similar levels as the normal group. -

Cerebral White Matter Lesions on Diffusion-Weighted Images
diagnostics Article Cerebral White Matter Lesions on Diffusion-Weighted Images and Delayed Neurological Sequelae after Carbon Monoxide Poisoning: A Prospective Observational Study Sangun Nah 1 , Sungwoo Choi 1, Han Bit Kim 1, Jungbin Lee 2, Sun-Uk Lee 3 , Young Hwan Lee 1, Gi Woon Kim 1 and Sangsoo Han 1,* 1 Department of Emergency Medicine, Soonchunhyang University Bucheon Hospital, Bucheon 14584, Korea; [email protected] (S.N.); [email protected] (S.C.); [email protected] (H.B.K.); [email protected] (Y.H.L.); [email protected] (G.W.K.) 2 Department of Radiology, Soonchunhyang University Bucheon Hospital, Bucheon 14584, Korea; [email protected] 3 Department of Neurology, Korea University Medical Center, Seoul 02841, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-32-621-5116 Received: 29 August 2020; Accepted: 14 September 2020; Published: 16 September 2020 Abstract: Introduction: Carbon monoxide (CO) poisoning can result in delayed neurological sequelae (DNS). Factors predicting DNS are still controversial. This study aims to determine whether acute brain lesions observed using diffusion-weighted magnetic resonance imaging (MRI) following acute CO poisoning are related to the subsequent development of DNS. Methods: This prospective study was conducted on patients with CO poisoning treated at a university hospital in Bucheon, Korea. From August 2016 to July 2019, a total of 283 patients visited the hospital because of CO poisoning. Exclusion criteria included age under 18 years, refusing hyperbaric oxygen therapy, refusing MRI, being discharged against medical advice, being lost to follow-up, having persistent neurological symptoms at discharge, and being transferred from another hospital 24 h after exposure. -

Fos Activation of Selective Afferents to Ventral Tegmental Area During Cue-Induced Reinstatement of Cocaine Seeking in Rats
The Journal of Neuroscience, September 19, 2012 • 32(38):13309–13325 • 13309 Behavioral/Systems/Cognitive Fos Activation of Selective Afferents to Ventral Tegmental Area during Cue-Induced Reinstatement of Cocaine Seeking in Rats Stephen V. Mahler and Gary S. Aston-Jones Department of Neurosciences, Medical University of South Carolina, Charleston, South Carolina 29425 Ventral tegmental area (VTA) dopamine neurons are crucial for appetitive responses to Pavlovian cues, including cue-induced reinstate- ment of drug seeking. However, it is unknown which VTA inputs help activate these neurons, transducing stimuli into salient cues that drive drug-seeking behavior. Here we examined 56 VTA afferents from forebrain and midbrain that are Fos activated during cue-induced reinstatement. We injected the retrograde tracer cholera toxin  subunit (CTb) unilaterally into rostral or caudal VTA of male rats. All animalsweretrainedtoself-administercocaine,thenextinguishedofthisbehavior.Onafinaltestday,animalswereexposedtoresponse- contingent cocaine-associated cues, extinction conditions, a non-cocaine-predictive CSϪ, or a novel environment, and brains were processed to visualize CTb and Fos immunoreactivity to identify VTA afferents activated in relation to behaviors. VTA-projecting neurons in subregions of medial accumbens shell, ventral pallidum, elements of extended amygdala, and lateral septum (but not pre- frontal cortex) were activated specifically during cue-induced cocaine seeking, and some of these were also activated proportionately to the degree of cocaine seeking. Surprisingly, though efferents from the lateral hypothalamic orexin field were also Fos activated during reinstatement, these were largely non-orexinergic. Also, VTA afferents from the rostromedial tegmental nucleus and lateral habenula were specifically activated during extinction and CSϪ tests, when cocaine was not expected. -

On-Line Table: MRI Imaging Recommendation and Summary Of
On-line Table: MRI imaging recommendation and summary of key features Sequence Pathologies Visible Key Features T1 volumetric high-resolution Lewy body dementia Less consistent pattern of cerebral volume loss; a pattern of whole-brain reformatted in relatively focused atrophy of the midbrain, hypothalamus, axial, coronal, and sagittal planes and substantia innominata, with a relative sparing of the hippocampus and temporoparietal cortex; relatively little cortical atrophy Posterior cortical atrophy Bilateral parieto-occipital and temporo-occipital atrophy Pituitary region Pituitary macroadenoma: mass lesion intrinsic to pituitary Ͼ10 mm; T1 hypointense to gray matter (may be heterogeneous if hemorrhage present), T2 isointense, enhancing solid components; may extend into suprasellar region to distort optic chiasm; laterally may invade cavernous sinus FLAIR, volumetric whole-brain Focal cortical dysplasia T2 hyperintense cortical lesions Seizure (posterior cortical) Blurring of gray-white matter junction Focal white matter abnormal signal Transmantle increased signal and abnormal gyral pattern Mesial temporal sclerosis, possibly others Primary brain tumors Both low- and high-grade gliomas usually have associated FLAIR abnormality, involving cortex and white matter Enhancement, diffusion restriction, elevated cerebral blood volume in higher grade lesions Metastases Location at gray-white matter junction Multiplicity Heterogeneous, depending on primary lesion, hemorrhage Enhancement, variable pattern Edema out of proportion to size of lesion -

A Bilateral Cortico-Striate Projection
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.28.1.71 on 1 February 1965. Downloaded from J. Neurol. Neurosurg. Psychiat., 1965, 28, 71 A bilateral cortico-striate projection J. B. CARMAN, W. M. COWAN, T. P. S. POWELL, AND K. E. WEBSTER From the Departments of Anatomy, University of Oxford, and University College, London During the course of studies on the projection of the ined, and evidence for a bilateral projection has been cerebral cortex upon the striatum in the rabbit found in 20 animals. The evidence for this projec- (Carman, Cowan, and Powell, 1963) and the cat tion depends upon the collective findings in several (Webster, 1964) degeneration was seen bilaterally in brains, but only a few typical examples will be Nauta preparations of the striatum in some, but not described in full. The findings in the remaining all, animals. For two main reasons this observation experiments will be summarized in composite was not included in the earlier study. First, because figures. of the difficulty of interpreting any findings of Experiment R30 is representative of the rabbit bilateral degeneration in silver preparations, and, brains in which a projection to the contralateral particularly as it is well known that the striatum striatum was found after a lesion involving the sen- commonly shows pseudo-degeneration, it was im- sori-motor cortex. The cortical damage in this brain perative to exclude this possibility by the prepara- is in the form of a broad strip along the dorsal guest. Protected by copyright. tion of further material using both the frozen and surface of the hemisphere from just behind the paraffin Nauta methods. -

Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G
Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G. Jamieson, M.D. Weill Cornell Medicine, New York, NY The patient who presents with either acute or subacute confusion, in the absence of a clearly defined speech disorder and focality on neurological examination that would indicate an underlying mass lesion, needs to be evaluated for a multitude of neurological conditions. Many of the conditions that produce the recent onset of alteration in mental status, that ranges from mild confusion to florid delirium, may be due to infectious or inflammatory conditions that warrant acute intervention such as antimicrobial drugs, steroids or plasma exchange. However, some patients with recent onset of confusion have an underlying toxic-metabolic disorders indicating a specific diagnosis with need for appropriate treatment. The clinical presentations of some patients may indicate the diagnosis (e.g. hypoglycemia, chronic alcoholism) while the imaging patterns must be recognized to make the diagnosis in other patients. Toxic-metabolic disorders constitute a group of diseases and syndromes with diverse causes and clinical presentations. Many toxic-metabolic disorders have no specific neuroimaging correlates, either at early clinical stages or when florid symptoms develop. However, some toxic-metabolic disorders have characteristic abnormalities on neuroimaging, as certain areas of the central nervous system appear particularly vulnerable to specific toxins and metabolic perturbations. Areas of particular vulnerability in the brain include: 1) areas of high-oxygen demand (e.g. basal ganglia, cerebellum, hippocampus), 2) the cerebral white matter and 3) the mid-brain. Brain areas of high-oxygen demand are particularly vulnerable to toxins that interfere with cellular respiratory metabolism. -

Rhesus Monkey Brain Atlas Subcortical Gray Structures
Rhesus Monkey Brain Atlas: Subcortical Gray Structures Manual Tracing for Hippocampus, Amygdala, Caudate, and Putamen Overview of Tracing Guidelines A) Tracing is done in a combination of the three orthogonal planes, as specified in the detailed methods that follow. B) Each region of interest was originally defined in the right hemisphere. The labels were then reflected onto the left hemisphere and all borders checked and adjusted manually when necessary. C) For the initial parcellation, the user used the “paint over function” of IRIS/SNAP on the T1 template of the atlas. I. Hippocampus Major Boundaries Superior boundary is the lateral ventricle/temporal horn in the majority of slices. At its most lateral extent (subiculum) the superior boundary is white matter. The inferior boundary is white matter. The anterior boundary is the lateral ventricle/temporal horn and the amygdala; the posterior boundary is lateral ventricle or white matter. The medial boundary is CSF at the center of the brain in all but the most posterior slices (where the medial boundary is white matter). The lateral boundary is white matter. The hippocampal trace includes dentate gyrus, the CA3 through CA1 regions of the hippocamopus, subiculum, parasubiculum, and presubiculum. Tracing A) Tracing is done primarily in the sagittal plane, working lateral to medial a. Locate the most lateral extent of the subiculum, which is bounded on all sides by white matter, and trace. b. As you page medially, tracing the hippocampus in each slice, the superior, anterior, and posterior boundaries of the hippocampus become the lateral ventricle/temporal horn. c. Even further medially, the anterior boundary becomes amygdala and the posterior boundary white matter. -

Uncinate Fasciculus Findings in Schizophrenia: a Magnetic Resonance Diffusion Tensor Imaging Study
Article Uncinate Fasciculus Findings in Schizophrenia: A Magnetic Resonance Diffusion Tensor Imaging Study Marek Kubicki, M.D., Ph.D. Objective: Disruptions in connectivity prominent white matter tract connecting between the frontal and temporal lobes temporal and frontal brain regions, in 15 Carl-Fredrik Westin, Ph.D. may explain some of the symptoms ob- patients with chronic schizophrenia and served in schizophrenia. Conventional 18 normal comparison subjects. A 1.5-T GE Stephan E. Maier, M.D., Ph.D. magnetic resonance imaging (MRI) stud- Echospeed system was used to acquire 4- ies, however, have not shown compelling mm-thick coronal line-scan diffusion ten- evidence for white matter abnormalities, sor images. Maps of the fractional anisot- Melissa Frumin, M.D. because white matter fiber tracts cannot ropy were generated to quantify the water be visualized by conventional MRI. Diffu- diffusion within the uncinate fasciculus. Paul G. Nestor, Ph.D. sion tensor imaging is a relatively new technique that can detect subtle white Results: Findings revealed a group-by- Dean F. Salisbury, Ph.D. matter abnormalities in vivo by assessing side interaction for fractional anisotropy the degree to which directionally orga- and for uncinate fasciculus area, derived from automatic segmentation. The pa- Ron Kikinis, M.D. nized fibers have lost their normal integ- rity. The first three diffusion tensor imag- tients with schizophrenia showed a lack of ing studies in schizophrenia showed lower normal left-greater-than-right asymmetry Ferenc A. Jolesz, M.D. anisotropic diffusion, relative to compari- seen in the comparison subjects. son subjects, in whole-brain white matter, Robert W. -

The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1935 The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat James K. L. Choy Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Anatomy Commons Recommended Citation Choy, James K. L., "The Embryology and Fiber Tract Connections of the Corpus Striatum in the Albino Rat" (1935). Master's Theses. 22. https://ecommons.luc.edu/luc_theses/22 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1935 James K. L. Choy LOYOLA UNIVERSITY SCHOOl, OF MEDICINE THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. A THESIS SUBMITTED TO THE FACULTY of the GRADUATE SCHOOL of LOYOLA UNIVERSITY IN CANDIDACY FOR THE DEGREE OF MASTER OF SCIENCE by James K.L. Choy, B.S.M. 1935 THE EMBRYOLOGY AND FIBER TRACT CONNECTIONS OF THE CORPUS STRIATUM IN THE ALBINO RAT. I. PREFACE Before entering upon a discussion of the problem itself, I would lil{e to take this opportunity to acknowledge the assis tance and encouragement I received in the preparation of this paper. To Dr. R. M. Strong, who suggested the problem, I am deeply obligated for his encouragement, practical guidance, and helpful suggestions in the procedure of this work. -

Intersection of Structural and Functional Connectivity of the Nucleus Basalis of Meynert in Parkinson's Disease Dementia and L
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.27.221853; this version posted July 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Intersection of structural and functional connectivity of the nucleus basalis of Meynert in Parkinson’s disease dementia and Lewy body dementia. Authors: Ashwini Oswala,b,c*†, James Gratwicked†, Harith Akramd, Marjan Jahanshahid, Laszlo Zaborszkye, Peter Browna,b , Marwan Harizd,f, Ludvic Zrinzod, Tom Foltynied, Vladimir Litvakc †Denotes equal contribution to Authorship Affiliations: a Medical Research Brain Network Dynamics Unit, University of Oxford, Oxford, UK b Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, UK c Wellcome Trust Centre for Neuroimaging, UCL Institute of Neurology, 12 Queen Square, London, UK d Department of Clinical & Movement Neurosciences, UCL Institute of Neurology and The National Hospital for Neurology and Neurosurgery, Queen Square, London, UK e Center for Molecular and Behavioral Neuroscience, Rutgers University, USA f Department of Clinical Neuroscience, Umeå University, Umeå, Sweden To whom correspondence should be addressed: [email protected] or [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.07.27.221853; this version posted July 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -
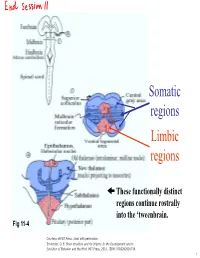
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G.