An Investigation of Acid Sulfate Soils in the Logan-Coomera Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Planning Scheme Maps 3 of 16
Map Number Map Name Currumbin Currumbin Valley Darlington Eagle Heights Gilberton Gilston Hollywell Jacobs Well Lower Beechmont Maudsland Mermaid Beach Mount Cougal Mount Tamborine Mount Wunburra Mudgeeraba Nerang Natural Bridge Ormeau Oxenford Pages Pinnacle Pimpama Shaws Pocket Short Island Slacks Creek Southport Springbrook Stephens Swamp Surfers Paradise Tallebudgera The Bedroom Tweed Heads Tyalgum Upper Coomera Waterford Wolffdene Woogoompah Worongary Woongoolba Ver.1.2 List of Planning Scheme Maps 3 of 16 5.0 Emerging Communities Domain – Structure Plan Maps The Structure Plan Maps are located in Part 5 – Domains of the hardcopy version of this Planning Scheme. They are also located on the Gold Coast Planning Scheme 2003 CD that supports this version or can be viewed online at goldcoastcity.com.au/planningscheme. Map Number Map Name EC1 Beenleigh District Structure Plan EC2 Albert Corridor A: Ormeau Structure Plan EC3 Albert Corridor B: Upper Coomera Structure Plan EC4 Albert Corridor C: Otmoor Road Structure Plan – Map Withdrawn EC5 Albert Corridor D: South Helensvale Structure Plan EC6 Albert Corridor E: Kopps Road Structure Plan EC7 Gilston Structure Plan EC8 Reedy Creek Structure Plan EC9 Inter-Urban Break Structure Plan 6.0 Local Area Plan Maps The Local Area Plan Maps are located in Part 6 – Local Area Plans of the hardcopy version of this Planning Scheme. They are also located on the Gold Coast Planning Scheme 2003 CD that supports this version or can be viewed online at goldcoastcity.com.au/planningscheme. Note: Within the Planning Scheme, reference to a Local Area Plan should be taken to include reference to a Structure Plan as defined by s.137 of the Sustainable Planning Act 2009. -

Drivers for Decentralised Systems in South East Queensland
Drivers for Decentralised Systems in South East Queensland Grace Tjandraatmadja, Stephen Cook, Angel Ho, Ashok Sharma and Ted Gardner October 2009 Urban Water Security Research Alliance Technical Report No. 13 Urban Water Security Research Alliance Technical Report ISSN 1836-5566 (Online) Urban Water Security Research Alliance Technical Report ISSN 1836-5558 (Print) The Urban Water Security Research Alliance (UWSRA) is a $50 million partnership over five years between the Queensland Government, CSIRO’s Water for a Healthy Country Flagship, Griffith University and The University of Queensland. The Alliance has been formed to address South-East Queensland's emerging urban water issues with a focus on water security and recycling. The program will bring new research capacity to South-East Queensland tailored to tackling existing and anticipated future issues to inform the implementation of the Water Strategy. For more information about the: UWSRA - visit http://www.urbanwateralliance.org.au/ Queensland Government - visit http://www.qld.gov.au/ Water for a Healthy Country Flagship - visit www.csiro.au/org/HealthyCountry.html The University of Queensland - visit http://www.uq.edu.au/ Griffith University - visit http://www.griffith.edu.au/ Enquiries should be addressed to: The Urban Water Security Research Alliance PO Box 15087 CITY EAST QLD 4002 Ph: 07-3247 3005; Fax: 07-3405 3556 Email: [email protected] Ashok Sharma - Project Leader Decentralised Systems CSIRO Land and Water 37 Graham Road HIGHETT VIC 3190 Ph: 03-9252 6151 Email: [email protected] Citation: Grace Tjandraatmadja, Stephen Cook, Angel Ho, Ashok Sharma and Ted Gardner (2009). Drivers for Decentralised Systems in South East Queensland. -

Pimpama River Catchment Hydrological Study Addendum Report
Pimpama River Catchment Hydrological Study Addendum Report July 2015 Title: Pimpama River Catchment Hydrological Study - Addendum Report 2015 Author: Study for: City Planning Branch Planning and Environment Directorate The City of Gold Coast File Reference: WF18/44/02 (P3) TRACKS #50622520 Version history Changed by Reviewed by & Version Comments/Change & date date 1.0 Adoption of BOM’s new IFD 2013 2.0 Grammar Review Distribution list Name Title Directorate Branch NH Team PE City Planning TRACKS-#50622520-v3-PIMPAMA_RIVER_HYDROLOGICAL_STUDY_ADDENDUM_REPORT_JULY_2015 Page 2 of 26 1. Executive Summary The City of Gold Coast (City) undertook a hydrological study for Pimpama River catchment in December 2014 (City 2014, Ref 1). In the study, the Pimpama River catchment hydrological model was developed using the URBS modelling software. The model was calibrated to three historical flood events and verified against another four flood events. The design rainfalls from 2 to 2000 year annual recurrence intervals (ARIs) of the study were based on study undertaken by Australian Water Engineering (AWE) in 1998 and CRC-FORGE. In early 2015, the Bureau of Meteorology (BOM) released new IFD (2013) design rainfalls as part of the revision of Engineers Australia’s design handbook ‘Australian Rainfall and Runoff: A Guide to Flood Estimation’. In July 2015, the 2014 calibrated Pimpama hydrological model was used to run the design events using rainfall data obtained from the BOM’s new 2013 IFD tables. This report documents the review of City 2014 model and should be read in conjunction with the City 2014 hydrological study report. The original forest factor, catchment and channel parameters obtained from the 2014 calibrated Pimpama hydrological model were used for this study update. -

Gold Coast in Queensland
Gold Coast in Queensland Gold Coast — one of the fastest growing Australian cities. Gold Coast is a coastal town in Queensland, in eastern Australia, famous for its line of beautiful beaches. With easy connectivity to Brisbane, Gold Coast is one of the most popular tourist spots in all of Australia. No wonder then, the city has the highest population among non-capital cities of Australia, and its humble airport is the sixth busiest airport in Australia. Southern Gold Coast offers a laid back atmosphere in comparison to the frenzied fun of Surfer's Paradise. Gold Coast Attractions The attractions at Gold Coast are numerous; they include beaches, hinterlands, and posh precincts. Beaches:The Gold Coast is lined with numerous beaches- thirty five in all. Main Beach: This is actually the main surf beach of the town. Pavilion 34, which used to be a bathing pavilion, now serves as a casual cafe serving some appetizing chillo rolls and potato scallops. Surfer's Paradise: Surfer's Paradise alone makes Gold Coast worth a visit. Thanks to its shopping centers, arcades, a beachfront promenade lined with more than a hundred stalls, and above all, some of the best sea-breaks in the world. A long stretch of golden sand is good for a walk or simply to soak up some sun. Surfer's Paradise has a pulsating nightlife too. An array of nightclubs, restaurants and pubs- all clustered within walkable distance of one another remain open till the wee hours of the morning. Broadbeach: Broadbeach has really come into its own. Broadbeach offers an experience that is distinctly different from what Surfer's Paradise has to offer. -
Residential Density (Areas Outside Laps) - Overlay Map OM4 - Index (Ver
Residential Density (Areas outside LAPs) - Overlay Map OM4 - Index (Ver. 1.0) LOGANLOGAN CITYCITY REDLANDREDLAND SHIRESHIRE BETHANIABETHANIABETHANIA EAGLEBYEAGLEBYEAGLEBY EDENSEDENSEDENS LANDINGLANDINGLANDING ALBERTONALBERTONALBERTON WATERFORDWATERFORD 11 BEENLEIGHBEENLEIGHBEENLEIGH 22 HOLMVIEWHOLMVIEW MTMT WARRENWARREN STAPYLTONSTAPYLTONSTAPYLTON WOONGOOLBAWOONGOOLBA PARKPARKPARK BAHRSBAHRSBAHRS SCRUB SCRUBSCRUB BAHRSBAHRSBAHRS SCRUB SCRUBSCRUB STEIGLITZSTEIGLITZSTEIGLITZ GILBERTONGILBERTON WINDAROOWINDAROO YATALAYATALAYATALA BELIVAHBELIVAHBELIVAH BANNOCK-BANNOCK-BANNOCK- BURN BURNBURNBURN NORWELLNORWELL ORMEAUORMEAU WOLFFDENEWOLFFDENE ORMEAUORMEAU WOLFFDENEWOLFFDENE JACOBSJACOBSJACOBS WELL WELLWELL LUSCOMBELUSCOMBELUSCOMBE PIMPAMAPIMPAMAPIMPAMA SOUTHSOUTHSOUTH KINGSHOLMEKINGSHOLMEKINGSHOLME SOUTHSOUTHSOUTH STRADBROKESTRADBROKESTRADBROKE Coral ISLANDISLANDISLANDISLAND WILLOWWILLOW VALEVALE CEDARCEDAR CREEKCREEK WILLOWWILLOW VALEVALE CEDARCEDAR CREEKCREEK COOMERACOOMERA HOPEHOPE ISLANDISLAND UPPERUPPER COOMERACOOMERA UPPERUPPER COOMERACOOMERA PARADISEPARADISEPARADISE WONGAWALLANWONGAWALLAN PARADISEPARADISEPARADISE POINTPOINTPOINT HELENSVALEHELENSVALE HOLLYWELLHOLLYWELL 3OXENFORD3OXENFORD 44 55 RUNAWAYRUNAWAY BAYBAY COOMBABAHCOOMBABAH BIGGERABIGGERABIGGERA WATERS WATERSWATERS MAUDSLANDMAUDSLAND MAUDSLANDMAUDSLAND GAVENGAVEN ARUNDELARUNDELARUNDEL GUANABAGUANABA GAVENGAVEN LABRADORLABRADORLABRADOR PARKWOODPARKWOODPARKWOOD BEAUDESERTBEAUDESERT SHIRESHIRE 1717 66 77 MAINMAIN 1717 ERNESTERNEST6ERNEST6 77 BEACHBEACHBEACH MOLENDINARMOLENDINAR -

Surface Water Ambient Network (Water Quality) 2020-21
Surface Water Ambient Network (Water Quality) 2020-21 July 2020 This publication has been compiled by Natural Resources Divisional Support, Department of Natural Resources, Mines and Energy. © State of Queensland, 2020 The Queensland Government supports and encourages the dissemination and exchange of its information. The copyright in this publication is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) licence. Under this licence you are free, without having to seek our permission, to use this publication in accordance with the licence terms. You must keep intact the copyright notice and attribute the State of Queensland as the source of the publication. Note: Some content in this publication may have different licence terms as indicated. For more information on this licence, visit https://creativecommons.org/licenses/by/4.0/. The information contained herein is subject to change without notice. The Queensland Government shall not be liable for technical or other errors or omissions contained herein. The reader/user accepts all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this information. Summary This document lists the stream gauging stations which make up the Department of Natural Resources, Mines and Energy (DNRME) surface water quality monitoring network. Data collected under this network are published on DNRME’s Water Monitoring Information Data Portal. The water quality data collected includes both logged time-series and manual water samples taken for later laboratory analysis. Other data types are also collected at stream gauging stations, including rainfall and stream height. Further information is available on the Water Monitoring Information Data Portal under each station listing. -

SOUTH STRADBROKE ISLAND WATERS South Stradbroke Island Waters
SOUTH STRADBROKE ISLAND WATERS South Stradbroke Island Waters Situated only 15 minutes from the Coomera Waters Marina and 20 minutes from Runaway Bay, South Stradbroke Island Waters unquestionably provides the best of both worlds. You can live the serene island lifestyle you have always dreamed of – with the convenience of all the Gold Coast’s key amenities just a short boat ride away. Perfectly positioned next to Couran Cove, on the former site of Couran Point Resort, the estate offers large homesites. All lots are equipped with full pressure town water as well as natural gas and sewerage. You can live comfortably surrounded by true natural beauty. Wind your way through state forests, along walkways to the untouched beaches. Relax in the sand unencumbered by crowds. South Stradbroke Island Waters is undeniably a boat lover’s paradise. This exceptional island community surrounds a peaceful inlet that oers direct access to the Gold Coast Broadwater. This is undoubtedly the most exclusive island real estate in Queensland. With a limited number of waterfront allotments available, contact our Coomera Home and Land Centre today. Our Location Our COVENANT As one of the largest developers in South-East Queensland, QM Properties works to ensure that there are quality development guidelines employed for each of their communities. The high quality of the streetscapes, home designs and manicured gardens in QM estates are the result of our established Community Development Standards. Covenants have become an invaluable part of all modern, quality developments. Estate covenants are designed to ensure the high standard of our estates as well as work to protect buyers’ investments. -
Gold Coast Attractions Guide
GOLD COAST ATTRACTIONS GUIDE See more & save on Australia’s top attractions iventurecard.com iventurecard.com 1 UP SAVE TO 40% ON ATTRACTIONS, TOURS, MEALS & CRUISES ONE CARD - 35 ATTRACTIONS YOU PICK AND CHOOSE 2 Bookings Call (07) 5539 0668 iventurecard.com 3 TABLE OF CONTENTS UPGRADE OFFERS ATTRACTIONS LIST ATTRACTIONS LIST ATTRACTION iVENTURE CARD OFFER PAGE ATTRACTION UPGRADE OFFERS PAGE 7D Cinema Two Movies 8 Australian Kayaking $30pp ½ Day Dolphin & Adventures Stradbroke Island Tour 23 Aquaduck Safaris Land & Water Cruise Adventure 7 Dolphins in Paradise $55pp Moreton Island Australian Kayaking 2 Hour Sunset Tour or Cruise, Snorkelling & Lunch 24 Adventures 2 Hour Kayak Hire 8 Gold Coast Watersports $40pp 5 Minute Flyboard 24 Catch a Crab Catch a Crab Tour - Morning 9 Gold Coast Watersports $30pp Parasailing (min 2 pp) 25 Charlie’s Cafe & Bar Meal to the value of $35 9 Hanlan’s at Novotel Seafood Dinner Buffet Currumbin Wildlife Sanctuary Single Entry 10 $10pp 6.30pm - 9pm 25 Fire Truck Tours 1 Hour Tour on a Fire Truck 10 Hard Rock Cafe 3 Course Meal + Souvenir T-Shirt Get Wet Surf School 2 Hour Surf Lesson 11 or Pin* $20 Adult / $10 Child 26 $20pp Nocturnal Glow Gold Coast Wake Park 1 Hour Cable Pass on Main Lake 11 Southern Cross Day Tours Worm Tour 26 Gold Coast Watersports 30 Minute Jet ski (min 2 people) 12 Southern Cross Day Tours $20pp ½ Day Mt Tamborine Greyhound - Surfers Paradise Return Coach Transfer Morning Tour 27 to Brisbane or Byron Bay 12 Southern Cross Day Tours $20pp ½ Day Natural Bridge Hanlan’s at Novotel Seafood -
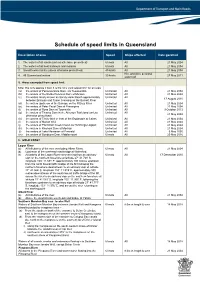
Schedule of Speed Limits in Queensland
Schedule of speed limits in Queensland Description of area Speed Ships affected Date gazetted 1. The waters of all canals (unless otherwise prescribed) 6 knots All 21 May 2004 2. The waters of all boat harbours and marinas 6 knots All 21 May 2004 3. Smooth water limits (unless otherwise prescribed) 40 knots All 21 May 2004 Hire and drive personal 4. All Queensland waters 30 knots 27 May 2011 watercraft 5. Areas exempted from speed limit Note: this only applies if item 3 is the only valid speed limit for an area (a) the waters of Perserverance Dam, via Toowoomba Unlimited All 21 May 2004 (b) the waters of the Bjelke Peterson Dam at Murgon Unlimited All 21 May 2004 (c) the waters locally known as Sandy Hook Reach approximately Unlimited All 17 August 2010 between Branyan and Tyson Crossing on the Burnett River (d) the waters upstream of the Barrage on the Fitzroy River Unlimited All 21 May 2004 (e) the waters of Peter Faust Dam at Proserpine Unlimited All 21 May 2004 (f) the waters of Ross Dam at Townsville Unlimited All 9 October 2013 (g) the waters of Tinaroo Dam in the Atherton Tableland (unless Unlimited All 21 May 2004 otherwise prescribed) (h) the waters of Trinity Inlet in front of the Esplanade at Cairns Unlimited All 21 May 2004 (i) the waters of Marian Weir Unlimited All 21 May 2004 (j) the waters of Plantation Creek known as Hutchings Lagoon Unlimited All 21 May 2004 (k) the waters in Kinchant Dam at Mackay Unlimited All 21 May 2004 (l) the waters of Lake Maraboon at Emerald Unlimited All 6 May 2005 (m) the waters of Bundoora Dam, Middlemount 6 knots All 20 May 2016 6. -
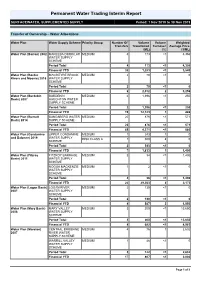
Permanent Water Trading Interim Report
Permanent Water Trading Interim Report SURFACEWATER, SUPPLEMENTED SUPPLY Period: 1 Nov 2019 to 30 Nov 2019 Transfer of Ownership – Water Allocations Water Plan Water Supply Scheme Priority Group Number Of Volume Volume Weighted Transfers Transferred Turnover Average Price (ML) (%) ($/ML) Water Plan (Barron) 2002 MAREEBA DIMBULAH MEDIUM 4 173 <1 4,356 WATER SUPPLY SCHEME Period Total 4 173 <1 4,356 Financial YTD 35 1,283 <1 3,540 Water Plan (Border MACINTYRE BROOK MEDIUM 2 70 <1 0 Rivers and Moonie) 2019 WATER SUPPLY SCHEME Period Total 2 70 <1 0 Financial YTD 6 2,012 2 3,259 Water Plan (Burdekin BURDEKIN MEDIUM 2 1,396 <1 250 Basin) 2007 HAUGHTON WATER SUPPLY SCHEME Period Total 2 1,396 <1 250 Financial YTD 19 12,123 1 486 Water Plan (Burnett BUNDABERG WATER MEDIUM 20 876 <1 571 Basin) 2014 SUPPLY SCHEME Period Total 20 876 <1 571 Financial YTD 69 4,271 <1 580 Water Plan (Condamine UPPER CONDAMINE MEDIUM 1 243 1 0 and Balonne) 2019 WATER SUPPLY RISK CLASS A 1 300 4 0 SCHEME Period Total 2 543 <1 0 Financial YTD 7 1,833 1 1,400 Water Plan (Fitzroy FITZROY BARRAGE MEDIUM 3 34 <1 1,400 Basin) 2011 WATER SUPPLY SCHEME NOGOA MACKENZIE MEDIUM 1 2 <1 0 WATER SUPPLY SCHEME Period Total 4 36 <1 1,400 Financial YTD 22 29,563 8 2,173 Water Plan (Logan Basin) LOGAN RIVER MEDIUM 2 130 <1 0 2007 WATER SUPPLY SCHEME Period Total 2 130 <1 0 Financial YTD 4 587 3 1,050 Water Plan (Mary Basin) MARY VALLEY MEDIUM 3 200 <1 13,650 2006 WATER SUPPLY SCHEME Period Total 3 200 <1 13,650 Financial YTD 8 682 <1 4,983 Water Plan (Moreton) CENTRAL BRISBANE MEDIUM -

Australasian Bulletin of Ecotoxicology and Environmental Chemistry
ABEEC New Cover 2015.pdf 1 23/07/2015 4:24:53 PM A a ustralasi Australasian Bulletin of Ecotoxicology and Environmental Chemistry The Ocial Bulletin of the Australasian Chapter of the Volume 2, 2015 Society of Environmental Toxicology and Chemistry Asia Pacific C M Y CM MY CY CMY K 1 AUSTRALASIAN BULLETIN OF ecOTOXICOLOGY & ENVIRONMENTAL CHEMISTRY Editor-in-Chief Dr Reinier Mann, Department of Science, Information Technology and Innovation, GPO Box 2454, Brisbane Qld 4000, Australia. email: [email protected]. Associate Editor Dr Anne Colville, Centre for Environmental Sustainability, University of Technology, Sydney, Ultimo, NSW 2007, Australia. email: [email protected]. Call for Papers The Bulletin welcomes Original Research Papers, Short Communications, Review Papers, Commentaries and Letters to the Editors. Guidelines for Authors For information on Guidelines for Authors please contact the editors. AIMS AND SCOPE The Australasian Bulletin of Ecotoxicology and Environmental Chemistry is a publication of the Australasian Chapter of the Society of Environmental Toxicology and Chemistry – Asia Pacific (a geographic unit of the Society of Environmental Toxicology and Chemistry). It is dedicated to publishing scientifically sound research articles dealing with all aspects of ecotoxicology and environmental chemistry. All data must be generated by statistically and analytically sound research, and all papers will be peer reviewed by at least two reviewers prior to being considered for publication. The Bulletin will give priority to the publication of original research that is undertaken on the systems and organisms of the Australasian and Asia-Pacific region, but papers will be accepted from anywhere in the world. -

Mangrove Wetlands
WETLAND MANAGEMENT PROFILE MANGROVE WETLANDS Mangrove wetlands are characterised by Description trees that are uniquely adapted to tolerate Mangroves are woody plants, usually trees (woodland daily or intermittent inundation by the sea. to forest) but also shrubs, growing in the intertidal Distinctive animals are nurtured in their zone and able to withstand periods of inundation by sheltered waters and mazes of exposed seawater each day. In this profile, the term ”mangrove mangrove roots; others live in the underlying wetland” is used to refer to wetlands that are characterised by dominance of mangroves. mud. A familiar sight on the coast throughout “Mangrove associates” are non-woody plants that Queensland, mangrove wetlands provide mainly or commonly occur in mangrove wetlands. frontline defence against storm damage, The terms “mangrove forest” and “mangrove swamp” enhance reef water quality and sustain are sometimes used in regard to particular types of fishing and tourism industries. These habitat mangrove wetland or to mangrove wetland in general. assets can be preserved and loss and degradation (through pollution and changed MANGROVE wetlands are characterised water flows) averted by raising awareness of by the dominance of mangroves — woody the benefits of mangrove wetlands and by implementing integrated plans for land and plants growing in the intertidal zone and resource use in the coastal zone. able to withstand periods of inundation by seawater each day. Situated in land zone 1, mangrove wetlands are invariably in the intertidal zone and sometimes extend narrowly for many kilometres inland along tidal rivers, also around some near-shore islands. Accordingly, they are below the level of Highest Astronomical Tide (HAT) and typically are in the lower, most frequently and deeply inundated parts of the intertidal zone.