Danger and Walsh (2008)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rivers Monitoring and Evaluation Plan V1.0 2020
i Rivers Monitoring and Evaluation Plan V1.0 2020 Contents Acknowledgement to Country ................................................................................................ 1 Contributors ........................................................................................................................... 1 Abbreviations and acronyms .................................................................................................. 2 Introduction ........................................................................................................................... 3 Background and context ........................................................................................................ 3 About the Rivers MEP ............................................................................................................. 7 Part A: PERFORMANCE OBJECTIVES ..................................................................................... 18 Habitat ................................................................................................................................. 24 Vegetation ............................................................................................................................ 29 Engaged communities .......................................................................................................... 45 Community places ................................................................................................................ 54 Water for the environment .................................................................................................. -

Action Statement No.134
Action statement No.134 Flora and Fauna Guarantee Act 1988 Yarra Pygmy Perch Nannoperca obscura © The State of Victoria Department of Environment, Land, Water and Planning 2015 This work is licensed under a Creative Commons Attribution 4.0 International licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo and the Department of Environment, Land, Water and Planning (DELWP) logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ Cover photo: Tarmo Raadik Compiled by: Daniel Stoessel ISBN: 978-1-74146-670-6 (pdf) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. Accessibility If you would like to receive this publication in an alternative format, please telephone the DELWP Customer Service Centre on 136 186, email [email protected], or via the National Relay Service on 133 677, email www.relayservice.com.au. This document is also available on the internet at www.delwp.vic.gov.au Action Statement No. 134 Yarra Pygmy Perch Nannoperca obscura Description The Yarra Pygmy Perch (Nannoperca obscura) fragmented and characterised by moderate levels is a small perch-like member of the family of genetic differentiation between sites, implying Percichthyidae that attains a total length of 75 mm poor dispersal ability (Hammer et al. -

Crustacea) from Australian Fresh Waters : Foundation Studies
Records of the Australian Museum (1988) Supplement 10. ISBN 0 7305 5856 8 1 The Taxonomy of Crangonyctoid Amphipoda (Crustacea) from Australian Fresh Waters : Foundation Studies W.D. WILLIAMS1 AND J.L. BARNARD2 lDepartment of Zoology, University of Adelaide, G.P.O. Box 498, Adelaide, SA 5001, Australia 2Smithsonian Institution, NHB-163, Washington, D.e. 20560, USA ABSTRACT. A review and inventory of all 26 previously described species of freshwater crangonyctoid amphipods in Australia is given and accompanied by the description of seven new species found mixed in type collections or otherwise associated. All extant types have been examined and redescribed. The Australian crangonyctoids belong in the families Paramelitidae and Neoniphargidae and a new family, Perthiidae. Four new genera are described to align the classification properly. The Australian crangonyctoids are now placed in the families Paramelitidae, with Austrogammarus, Austrocrangonyx, Antipodeus (n.gen.), Hurleya, Uroctena, Giniphargus and Protocrangonyx; the Neoniphargidae, with Tasniphargus (n.gen.), Neoniphargus, Yulia (n.gen.) and Wesniphargus (n.gen.); and Perthiidae, with Perthia. Keys are provided to (1) the world groups of crangonyctoids, (2) Australian crangonyctoids, (3) the genera of each family, and (4) individually for the species of each genus. Contents Introduction ......................................................................................................... 3 Methods of Dissection and Description ................ " .. , ........... " .. , . .. . -
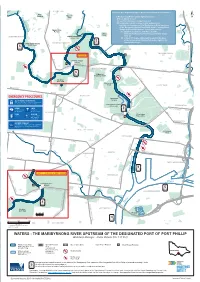
THE MARIBYRNONG RIVER UPSTREAM of the DESIGNATED PORT of PORT PHILLIP Waterway Manager - Parks Victoria (Ph: 131 963)
KEILOR EAST Exclusive Use & Special Purpose Areas for the Purpose of Clause 13. Allan Reserve Rosehill Maribyrnong ROAD Park a) Maribyrnong River- special light provisions N Creek A Recreational Vessel- (i) used for training or competition;ESSENDON and (ii) is not powered but is propelled by using oars or paddles; on the waters of the Maribyrnong River upstream of the Designated Port of Port Phillip to the Canning Street Moonee Monte Carlo Bridge shall exhibit between sunset and sunrise - Reserve (i) a light in accordance with Rule 25 of the MILLEARA Steele Clifton International Rules for Preventing Collisions at Sea, Park SUNSHINE NORTH LOWER MARIBYRNONG 1972; or RIVER LAND (ii) a fixed 180 degree white light located on the bow MILITARY of the vessel and a flashing 180 degree light on the Ponds AVONDALE HEIGHTS stern of the vessel. LOWER MARIBYRNONG RIVER LAND ABERFELDIE MOONEE PONDS CITYLINK See Inset A ROAD CANNING STREET BRIDGE CORDITE ROAD Creek RALEIGH AVENUE MARIBYRNONG STREET MARIBYRNONG ROAD ORMOND ROAD CANNING River Highpoint River Shopping Centre EPSOM Medway Golf Club ROAD Pipemakers Park ASCOT VALE BALLARAT ROAD HAMPSTEAD MAIDSTONE LANGS ROAD Thompson Reserve STREET BALLARAT LOWER MARIBYRNONG RIVER LAND FLEMINGTON AVENUE RACECOURSE ROAD ROAD BRAYBROOK ROAD Flemington FARNSWORTH Racecourse Creek SMITHFIELD KENSINGTON ROAD MACAULAY ROAD ASHLEY WEST FOOTSCRAY J J Holland Park SUNSHINE ROAD Stony FOOTSCRAY DYNON ROAD ROAD STREET DEMPSTER GEELONG TOTTENHAM ROAD Creek STREET SEDDON FOOTSCRAY Ponds KINGSVILLE CITYLINK ROAD ROAD -

Additions to and Revisions of the Amphipod (Crustacea: Amphipoda) Fauna of South Africa, with a List of Currently Known Species from the Region
Additions to and revisions of the amphipod (Crustacea: Amphipoda) fauna of South Africa, with a list of currently known species from the region Rebecca Milne Department of Biological Sciences & Marine Research Institute, University of CapeTown, Rondebosch, 7700 South Africa & Charles L. Griffiths* Department of Biological Sciences & Marine Research Institute, University of CapeTown, Rondebosch, 7700 South Africa E-mail: [email protected] (with 13 figures) Received 25 June 2013. Accepted 23 August 2013 Three species of marine Amphipoda, Peramphithoe africana, Varohios serratus and Ceradocus isimangaliso, are described as new to science and an additional 13 species are recorded from South Africa for the first time. Twelve of these new records originate from collecting expeditions to Sodwana Bay in northern KwaZulu-Natal, while one is an introduced species newly recorded from Simon’s Town Harbour. In addition, we collate all additions and revisions to the regional amphipod fauna that have taken place since the last major monographs of each group and produce a comprehensive, updated faunal list for the region. A total of 483 amphipod species are currently recognized from continental South Africa and its Exclusive Economic Zone . Of these, 35 are restricted to freshwater habitats, seven are terrestrial forms, and the remainder either marine or estuarine. The fauna includes 117 members of the suborder Corophiidea, 260 of the suborder Gammaridea, 105 of the suborder Hyperiidea and a single described representative of the suborder Ingolfiellidea. -

Victorian Environmental Flows Monitoring and Assessment Program (VEFMAP) Stage 6
Victorian Environmental Flows Monitoring and Assessment Program (VEFMAP) Stage 6 Project Update – 2018 Southern Victorian Rivers - Fish Background 2017/18 Survey Sites and Timing The Victorian Environmental Flows Monitoring and In 2017/18, surveys were undertaken to investigate Assessment Program (VEFMAP) was established by processes associated with KEQ 1 and 2 in the following the Victorian Government in 2005 to monitor and sites: assess ecosystem responses to environmental watering in priority rivers across Victoria. The program’s results • Immigration - the lower reaches of the Barwon, help inform decisions for environmental watering by Bunyip, Glenelg, Tarwin and Werribee rivers and Victoria’s Catchment Management Authorities (CMAs), Cardinia Creek (Sept-Dec 2017). Melbourne Water and the Victorian Environmental • Dispersal - the Glenelg and Moorabool rivers Water Holder (VEWH). Over the past 12 years, the (Jan-Feb 2018). information collected through VEFMAP has provided valuable data and informed significant changes to the • Distribution and recruitment - the Glenelg and program. VEFMAP is now in its sixth stage of delivery Thomson rivers (Feb-Mar 2018). and includes a strong focus on “intervention” or “flow event” type questions, for vegetation and fish. Fish Monitoring - Southern Victorian Rivers The core objective for fish monitoring in VEFMAP Stage 6 for coastal rivers is to examine the importance of environmental flows in promoting immigration, dispersal and subsequent recruitment of diadromous fish. There are two key evaluation questions for fish in coastal Victorian rivers, which were developed in collaboration with CMAs. KEQ 1 Do environmental flows enhance immigration of diadromous fishes in coastal streams? Figure 1: A juvenile (top) and adult (bottom) Tupong KEQ 2 Do environmental flows enhance dispersal, (Photo: ARI) distribution and recruitment of diadromous fishes in coastal streams? delwp.vic.gov.au VEFMAP Stage 6 Southern Victorian Rivers - Fish Methods January following a rain event in late December. -

Urban Biodiversity Strategy 2013-2023
Map of Boroondara's biodiversity corridors, biogeographical zones and biologically significant sites. Koonung Creek Corridor 3 4 BALWYN 2 6 5 8 NORTH 18 Yarra River 18 7 Eastern Freeway Map of Boroondara's biodiversity corridors, Corridor Glass Creek 9 35 Corridor biogeographical zones and biologically significant10 sites. 58 36 Bulleen Road 1 Doncaster Road MapMap of Boroondara's 1: Boroondara’s biodiversity biodiversity corridors, corridors, 40 Hyde Park KEW 11 39 Corridor 34 Balwyn North Corridor biogeographicalbiogeographical zones zones and and biologically biologically significant significant sites.sites EAST 33 Studley 41 32 Koonung Creek Corridor Park 3 57 Kew 12 Corridor4 37 38BALWYN 31 2 Belmore Road Studley Park Road 6 5 8 High StreetKoonungKEWNORTH Creek Corridor PrincessStreet 3 18 Yarra River 7 BALWYN 18 Glass Creek4Eastern Freeway Cotham Road Corridor 42 54 9 35 Corridor HAWTHORNBALWYN 2 Whitehorse Road 6 5 BalwynRoad 10 8 13 43 NORTH 58 56 53 36 BulleenEAST Road 1 18 44 Yarra River 18 7 Eastern Freeway Barkers RoadDoncaster Road 55 40Corridor Hyde Park KEW14Glass Creek 11 9 Corridor CorridorChurch Street 34 Balwyn North Corridor 10 39 35 DenmarkStreet CANTERBURY EAST4833 46 58 Studley 41 36 15 Bulleen Road 1 32 47 57 45 52 Park Doncaster Road CanterburySURREY Corridor 40 KewHyde Park KEW 11 12 39 CorridorCorridor 37 34 BalwynBurwood North Corridor Road HILLS EAST38 33 31 Belmore Road WarrigalRoad Studley Park Road 51 Canterbury Road Studley 41 32 Park High Street KEW 57 HAWTHORN KewPrincessStreet 16 BurkeRoad 12 Corridor -

The Future of the Yarra
the future of the Yarra ProPosals for a Yarra river Protection act the future of the Yarra A about environmental Justice australia environmental Justice australia (formerly the environment Defenders office, Victoria) is a not-for-profit public interest legal practice. funded by donations and independent of government and corporate funding, our legal team combines a passion for justice with technical expertise and a practical understanding of the legal system to protect our environment. We act as advisers and legal representatives to the environment movement, pursuing court cases to protect our shared environment. We work with community-based environment groups, regional and state environmental organisations, and larger environmental NGos. We also provide strategic and legal support to their campaigns to address climate change, protect nature and defend the rights of communities to a healthy environment. While we seek to give the community a powerful voice in court, we also recognise that court cases alone will not be enough. that’s why we campaign to improve our legal system. We defend existing, hard-won environmental protections from attack. at the same time, we pursue new and innovative solutions to fill the gaps and fix the failures in our legal system to clear a path for a more just and sustainable world. envirojustice.org.au about the Yarra riverkeePer association The Yarra Riverkeeper Association is the voice of the River. Over the past ten years we have established ourselves as the credible community advocate for the Yarra. We tell the river’s story, highlighting its wonders and its challenges. We monitor its health and activities affecting it. -

Rivers and Streams Special Investigation Final Recommendations
LAND CONSERVATION COUNCIL RIVERS AND STREAMS SPECIAL INVESTIGATION FINAL RECOMMENDATIONS June 1991 This text is a facsimile of the former Land Conservation Council’s Rivers and Streams Special Investigation Final Recommendations. It has been edited to incorporate Government decisions on the recommendations made by Order in Council dated 7 July 1992, and subsequent formal amendments. Added text is shown underlined; deleted text is shown struck through. Annotations [in brackets] explain the origins of the changes. MEMBERS OF THE LAND CONSERVATION COUNCIL D.H.F. Scott, B.A. (Chairman) R.W. Campbell, B.Vet.Sc., M.B.A.; Director - Natural Resource Systems, Department of Conservation and Environment (Deputy Chairman) D.M. Calder, M.Sc., Ph.D., M.I.Biol. W.A. Chamley, B.Sc., D.Phil.; Director - Fisheries Management, Department of Conservation and Environment S.M. Ferguson, M.B.E. M.D.A. Gregson, E.D., M.A.F., Aus.I.M.M.; General Manager - Minerals, Department of Manufacturing and Industry Development A.E.K. Hingston, B.Behav.Sc., M.Env.Stud., Cert.Hort. P. Jerome, B.A., Dip.T.R.P., M.A.; Director - Regional Planning, Department of Planning and Housing M.N. Kinsella, B.Ag.Sc., M.Sci., F.A.I.A.S.; Manager - Quarantine and Inspection Services, Department of Agriculture K.J. Langford, B.Eng.(Ag)., Ph.D , General Manager - Rural Water Commission R.D. Malcolmson, M.B.E., B.Sc., F.A.I.M., M.I.P.M.A., M.Inst.P., M.A.I.P. D.S. Saunders, B.Agr.Sc., M.A.I.A.S.; Director - National Parks and Public Land, Department of Conservation and Environment K.J. -

The Dandenong Creek Catchment Extends from the Dandenong
The many dedicated groups already working in the catchment the are limited in their ability to deal with the scale of works necessary to improve the condition of the area. promise A coordinated approach is needed to shape future urban and the vision natural environments so they set standards for and attract the most sustainable 21st century investments and development. iving Links will establish an interconnected web of habitat corridors, linking existing open space, conservation reserves, recreation L facilities and the many fragmented patches of native vegetation across the catchment. It will the enhance the social and economic attractiveness of the region’s rapidly developing commercial, industrial and new residential centres by strengthening their present relationship with the natural environment. Fortunately, many of the basic building blocks needed to help turn the vision into reality already exist. The he Dandenong Creek catchment extends from the Dandenong catchment contains numerous regionally significant Ranges National Park near Kilsyth, sweeps south to include parks such as Jells Park, Shepherd’s Bush and the the rapidly growing industrial and urban areas surrounding Bushy Park Wetlands. These are well connected and T Greater Dandenong and Casey, before flowing south-west to provide a network of walking and cycling trails. link with Port Phillip Bay near Frankston. The catchment is also home to other state and regionally significant natural parklands such as In all, it covers 855 square kilometres of Melbourne’s south-east Braeside Park, Lysterfield Park, Churchill National Park region. Scattered throughout the catchment are a series of regional, and the Seaford-Edithvale Wetlands that are presently state, national and internationally significant natural features including relatively isolated from one another. -

Walk-Issue14-1963.Pdf
1963 Terms and Conditions of Use Copies of Walk magazine are made available under Creative Commons - Attribution Non-Commercial Share Alike copyright. Use of the magazine. You are free: • To Share- to copy, distribute and transmit the work • To Remix- to adapt the work Under the following conditions (unless you receive prior written authorisation from Melbourne Bushwalkers Inc.): • Attribution- You must attribute the work (but not in any way that suggests that Melbourne Bushwalkers Inc. endorses you or your use of the work). • Noncommercial- You may not use this work for commercial purposes. • Share Alike- If you alter, transform, or build upon this work, you may distribute the resulting work only under the same or similar license to this one. Disclaimer of Warranties and Limitations on Liability. Melbourne Bushwalkers Inc. makes no warranty as to the accuracy or completeness of any content of this work. Melbourne Bushwalkers Inc. disclaims any warranty for the content, and will not be liable for any damage or loss resulting from the use of any content. ----···············------------------------------· • BUSHWALKING • CAVING • ROCK CLIMBING • CAMPING • SKI TOURING PROVIDE A CHALLENGE TO MAN AND HIS EQUIPMENT, FOR OVER 30 YEARS, PADDYMADE CAMP GEAR HAS PROVED ITS WORTH TO THOUSANDS OF WALKERS AND OUT-OF-DOORS ADVEN TURERS. MAKE SURE YOU, TOO, HAVE THE BEST OF GEAR. From- PADDY PALLIN Py. ltd. 201 CASTLEREAGH STREET, SYDNEY - Phone BM 2685 Ask for our Latest Price List Get your copy of "Bushwalking - --- and Camping," by Paddy Pallin -5/6 posted --------------------------------------------------· CWalk A JOURNAL OF THE MELBOURNE BUSHW ALKERS NUMBER FOURTEEN 1963 CONTENTS: * BY THE PEOPLE 'l ... -
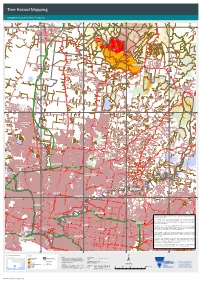
Tree Hazard Mapping
Tree Hazard Mapping Kangaroo Ground L3 ICC Footprint 320000.000000 330000.000000 340000.000000 350000.000000 0 0 0 0 0 0 0 0 0 0 0 0 . 0 0 0 0 N 0 0 O 0 0 R 6 6 T 8 8 H 5 5 E R N H I G H W A Y WALLAN Wattle Gully Y A W H G I Dry Creek H N R E H T R O N Chyser Creek Stony Creek Toorourrong Reservoir KINGLAKE WEST Johnson Creek Porcupine Gully Creek Halse Creek Scrubby Creek Pheasant Creek WHITTLESEA Number Three Creek Merri Creek Number One Creek KINGLAKE Recycle Dam Y A W E E R F E M U H Yan Yean Reservoir 0 0 0 0 0 0 0 0 0 0 0 0 . 0 0 0 0 0 0 0 Darebin Creek 0 4 4 8 8 5 Malcolm Creek 5 Aitken Creek CRAIGIEBURN ST ANDREWS Plenty River H U M E H I G H W A Y H U M E H I Red Shirt Gully Creek G H Greenvale Reservoir W A Y St Clair Reservoir HURSTBRIDGE Brodie Lake The Shankland Reservoir EPPING CHRISTMAS HILLS Yuroke Creek Sugarloaf Reservoir Blue Lake DIAMOND CREEK T U L THOMASTOWN L A M A METR R OPOLITAN RIN IN G ROAD E BROADMEADOWS F D R A E O E D R W G ROA A N RIN Y LITA T Y TROPO S ME N E Y L D P N E Y R O A TULLAMARINE D D A S D O Y A R D O GREENSBOROUGH G N R N I E Y R Y T N N R A R E I E O L R T P A P S E D O W R T D R I V D Edwardes Lake E A O ELTHAM R Biological Lake D G Y N A I A Upper Lake O R W R N R E H E E T G S R U E F W O E R N I O R Sports Fields Lake B A S M N A E L E L R C U A G LDE T R F RE EW WARRANDYTE AY CALDER FREEWAY Chirnside Park Drain COBURG PRESTON 0 0 0 0 0 0 0 0 0 Mullum Mullum Creek 0 0 0 .