DNA Spiral Supercoiling and Intramolecular Topological Interlink
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study of Spiral Transition Curves As Related to the Visual Quality of Highway Alignment
A STUDY OF SPIRAL TRANSITION CURVES AS RELA'^^ED TO THE VISUAL QUALITY OF HIGHWAY ALIGNMENT JERRY SHELDON MURPHY B, S., Kansas State University, 1968 A MJvSTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Civil Engineering KANSAS STATE UNIVERSITY Manhattan, Kansas 1969 Approved by P^ajQT Professor TV- / / ^ / TABLE OF CONTENTS <2, 2^ INTRODUCTION 1 LITERATURE SEARCH 3 PURPOSE 5 SCOPE 6 • METHOD OF SOLUTION 7 RESULTS 18 RECOMMENDATIONS FOR FURTHER RESEARCH 27 CONCLUSION 33 REFERENCES 34 APPENDIX 36 LIST OF TABLES TABLE 1, Geonetry of Locations Studied 17 TABLE 2, Rates of Change of Slope Versus Curve Ratings 31 LIST OF FIGURES FIGURE 1. Definition of Sight Distance and Display Angle 8 FIGURE 2. Perspective Coordinate Transformation 9 FIGURE 3. Spiral Curve Calculation Equations 12 FIGURE 4. Flow Chart 14 FIGURE 5, Photograph and Perspective of Selected Location 15 FIGURE 6. Effect of Spiral Curves at Small Display Angles 19 A, No Spiral (Circular Curve) B, Completely Spiralized FIGURE 7. Effects of Spiral Curves (DA = .015 Radians, SD = 1000 Feet, D = l** and A = 10*) 20 Plate 1 A. No Spiral (Circular Curve) B, Spiral Length = 250 Feet FIGURE 8. Effects of Spiral Curves (DA = ,015 Radians, SD = 1000 Feet, D = 1° and A = 10°) 21 Plate 2 A. Spiral Length = 500 Feet B. Spiral Length = 1000 Feet (Conpletely Spiralized) FIGURE 9. Effects of Display Angle (D = 2°, A = 10°, Ig = 500 feet, = SD 500 feet) 23 Plate 1 A. Display Angle = .007 Radian B. Display Angle = .027 Radiaji FIGURE 10. -

Construction Surveying Curves
Construction Surveying Curves Three(3) Continuing Education Hours Course #LS1003 Approved Continuing Education for Licensed Professional Engineers EZ-pdh.com Ezekiel Enterprises, LLC 301 Mission Dr. Unit 571 New Smyrna Beach, FL 32170 800-433-1487 [email protected] Construction Surveying Curves Ezekiel Enterprises, LLC Course Description: The Construction Surveying Curves course satisfies three (3) hours of professional development. The course is designed as a distance learning course focused on the process required for a surveyor to establish curves. Objectives: The primary objective of this course is enable the student to understand practical methods to locate points along curves using variety of methods. Grading: Students must achieve a minimum score of 70% on the online quiz to pass this course. The quiz may be taken as many times as necessary to successful pass and complete the course. Ezekiel Enterprises, LLC Section I. Simple Horizontal Curves CURVE POINTS Simple The simple curve is an arc of a circle. It is the most By studying this course the surveyor learns to locate commonly used. The radius of the circle determines points using angles and distances. In construction the “sharpness” or “flatness” of the curve. The larger surveying, the surveyor must often establish the line of the radius, the “flatter” the curve. a curve for road layout or some other construction. The surveyor can establish curves of short radius, Compound usually less than one tape length, by holding one end Surveyors often have to use a compound curve because of the tape at the center of the circle and swinging the of the terrain. -

The Ordered Distribution of Natural Numbers on the Square Root Spiral
The Ordered Distribution of Natural Numbers on the Square Root Spiral - Harry K. Hahn - Ludwig-Erhard-Str. 10 D-76275 Et Germanytlingen, Germany ------------------------------ mathematical analysis by - Kay Schoenberger - Humboldt-University Berlin ----------------------------- 20. June 2007 Abstract : Natural numbers divisible by the same prime factor lie on defined spiral graphs which are running through the “Square Root Spiral“ ( also named as “Spiral of Theodorus” or “Wurzel Spirale“ or “Einstein Spiral” ). Prime Numbers also clearly accumulate on such spiral graphs. And the square numbers 4, 9, 16, 25, 36 … form a highly three-symmetrical system of three spiral graphs, which divide the square-root-spiral into three equal areas. A mathematical analysis shows that these spiral graphs are defined by quadratic polynomials. The Square Root Spiral is a geometrical structure which is based on the three basic constants: 1, sqrt2 and π (pi) , and the continuous application of the Pythagorean Theorem of the right angled triangle. Fibonacci number sequences also play a part in the structure of the Square Root Spiral. Fibonacci Numbers divide the Square Root Spiral into areas and angle sectors with constant proportions. These proportions are linked to the “golden mean” ( golden section ), which behaves as a self-avoiding-walk- constant in the lattice-like structure of the square root spiral. Contents of the general section Page 1 Introduction to the Square Root Spiral 2 2 Mathematical description of the Square Root Spiral 4 3 The distribution -

Topography of the Histone Octamer Surface: Repeating Structural Motifs Utilized in the Docking of Nucleosomal
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 10489-10493, November 1993 Biochemistry Topography of the histone octamer surface: Repeating structural motifs utilized in the docking of nucleosomal DNA (histone fold/helix-strand-helix motif/parallel fi bridge/binary DNA binding sites/nucleosome) GINA ARENTS* AND EVANGELOS N. MOUDRIANAKIS*t *Department of Biology, The Johns Hopkins University, Baltimore, MD 21218; and tDepartment of Biology, University of Athens, Athens, Greece Communicated by Christian B. Anfinsen, August 5, 1993 ABSTRACT The histone octamer core of the nucleosome is interactions. The model offers strong predictive criteria for a protein superhelix offour spirally arrayed histone dimers. The structural and genetic biology. cylindrical face of this superhelix is marked by intradimer and interdimer pseudodyad axes, which derive from the nature ofthe METHODS histone fold. The histone fold appears as the result of a tandem, parallel duplication of the "helix-strand-helix" motif. This The determination of the structure of the histone octamer at motif, by its occurrence in the four dimers, gives rise torepetitive 3.1 A has been described (3). The overall shape and volume structural elements-i.e., the "parallel 13 bridges" and the of this tripartite structure is in agreement with the results of "paired ends of helix I" motifs. A preponderance of positive three independent studies based on differing methodolo- charges on the surface of the octamer appears as a left-handed gies-i.e., x-ray diffraction, neutron diffraction, and electron spiral situated at the expected path of the DNA. We have microscopic image reconstruction (4-6). Furthermore, the matched a subset of DNA pseudodyads with the octamer identification of the histone fold, a tertiary structure motif of pseudodyads and thus have built a model of the nucleosome. -

Some Curves and the Lengths of Their Arcs Amelia Carolina Sparavigna
Some Curves and the Lengths of their Arcs Amelia Carolina Sparavigna To cite this version: Amelia Carolina Sparavigna. Some Curves and the Lengths of their Arcs. 2021. hal-03236909 HAL Id: hal-03236909 https://hal.archives-ouvertes.fr/hal-03236909 Preprint submitted on 26 May 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Some Curves and the Lengths of their Arcs Amelia Carolina Sparavigna Department of Applied Science and Technology Politecnico di Torino Here we consider some problems from the Finkel's solution book, concerning the length of curves. The curves are Cissoid of Diocles, Conchoid of Nicomedes, Lemniscate of Bernoulli, Versiera of Agnesi, Limaçon, Quadratrix, Spiral of Archimedes, Reciprocal or Hyperbolic spiral, the Lituus, Logarithmic spiral, Curve of Pursuit, a curve on the cone and the Loxodrome. The Versiera will be discussed in detail and the link of its name to the Versine function. Torino, 2 May 2021, DOI: 10.5281/zenodo.4732881 Here we consider some of the problems propose in the Finkel's solution book, having the full title: A mathematical solution book containing systematic solutions of many of the most difficult problems, Taken from the Leading Authors on Arithmetic and Algebra, Many Problems and Solutions from Geometry, Trigonometry and Calculus, Many Problems and Solutions from the Leading Mathematical Journals of the United States, and Many Original Problems and Solutions. -

Archimedean Spirals ∗
Archimedean Spirals ∗ An Archimedean Spiral is a curve defined by a polar equation of the form r = θa, with special names being given for certain values of a. For example if a = 1, so r = θ, then it is called Archimedes’ Spiral. Archimede’s Spiral For a = −1, so r = 1/θ, we get the reciprocal (or hyperbolic) spiral. Reciprocal Spiral ∗This file is from the 3D-XploreMath project. You can find it on the web by searching the name. 1 √ The case a = 1/2, so r = θ, is called the Fermat (or hyperbolic) spiral. Fermat’s Spiral √ While a = −1/2, or r = 1/ θ, it is called the Lituus. Lituus In 3D-XplorMath, you can change the parameter a by going to the menu Settings → Set Parameters, and change the value of aa. You can see an animation of Archimedean spirals where the exponent a varies gradually, from the menu Animate → Morph. 2 The reason that the parabolic spiral and the hyperbolic spiral are so named is that their equations in polar coordinates, rθ = 1 and r2 = θ, respectively resembles the equations for a hyperbola (xy = 1) and parabola (x2 = y) in rectangular coordinates. The hyperbolic spiral is also called reciprocal spiral because it is the inverse curve of Archimedes’ spiral, with inversion center at the origin. The inversion curve of any Archimedean spirals with respect to a circle as center is another Archimedean spiral, scaled by the square of the radius of the circle. This is easily seen as follows. If a point P in the plane has polar coordinates (r, θ), then under inversion in the circle of radius b centered at the origin, it gets mapped to the point P 0 with polar coordinates (b2/r, θ), so that points having polar coordinates (ta, θ) are mapped to points having polar coordinates (b2t−a, θ). -
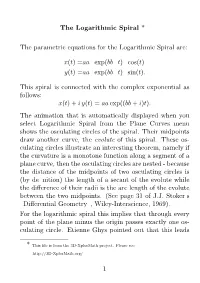
The Logarithmic Spiral * the Parametric Equations for The
The Logarithmic Spiral * The parametric equations for the Logarithmic Spiral are: x(t) =aa exp(bb t) cos(t) · · · y(t) =aa exp(bb t) sin(t). · · · This spiral is connected with the complex exponential as follows: x(t) + i y(t) = aa exp((bb + i)t). The animation that is automatically displayed when you select Logarithmic Spiral from the Plane Curves menu shows the osculating circles of the spiral. Their midpoints draw another curve, the evolute of this spiral. These os- culating circles illustrate an interesting theorem, namely if the curvature is a monotone function along a segment of a plane curve, then the osculating circles are nested - because the distance of the midpoints of two osculating circles is (by definition) the length of a secant of the evolute while the difference of their radii is the arc length of the evolute between the two midpoints. (See page 31 of J.J. Stoker’s “Differential Geometry”, Wiley-Interscience, 1969). For the logarithmic spiral this implies that through every point of the plane minus the origin passes exactly one os- culating circle. Etienne´ Ghys pointed out that this leads * This file is from the 3D-XplorMath project. Please see: http://3D-XplorMath.org/ 1 to a surprise: The unit tangent vectors of the osculating circles define a vector field X on R2 0 – but this vec- tor field has more integral curves, i.e.\ {solution} curves of the ODE c0(t) = X(c(t)), than just the osculating circles, namely also the logarithmic spiral. How is this compatible with the uniqueness results of ODE solutions? Read words backwards for explanation: eht dleifrotcev si ton ztihcspiL gnola eht evruc. -

Chapter 6 Protein Structure and Folding
Chapter 6 Protein Structure and Folding 1. Secondary Structure 2. Tertiary Structure 3. Quaternary Structure and Symmetry 4. Protein Stability 5. Protein Folding Myoglobin Introduction 1. Proteins were long thought to be colloids of random structure 2. 1934, crystal of pepsin in X-ray beam produces discrete diffraction pattern -> atoms are ordered 3. 1958 first X-ray structure solved, sperm whale myoglobin, no structural regularity observed 4. Today, approx 50’000 structures solved => remarkable degree of structural regularity observed Hierarchy of Structural Layers 1. Primary structure: amino acid sequence 2. Secondary structure: local arrangement of peptide backbone 3. Tertiary structure: three dimensional arrangement of all atoms, peptide backbone and amino acid side chains 4. Quaternary structure: spatial arrangement of subunits 1) Secondary Structure A) The planar peptide group limits polypeptide conformations The peptide group ha a rigid, planar structure as a consequence of resonance interactions that give the peptide bond ~40% double bond character The trans peptide group The peptide group assumes the trans conformation 8 kJ/mol mire stable than cis Except Pro, followed by cis in 10% Torsion angles between peptide groups describe polypeptide chain conformations The backbone is a chain of planar peptide groups The conformation of the backbone can be described by the torsion angles (dihedral angles, rotation angles) around the Cα-N (Φ) and the Cα-C bond (Ψ) Defined as 180° when extended (as shown) + = clockwise, seen from Cα Not -

And Beta-Helical Protein Motifs
Soft Matter Mechanical Unfolding of Alpha- and Beta-helical Protein Motifs Journal: Soft Matter Manuscript ID SM-ART-10-2018-002046.R1 Article Type: Paper Date Submitted by the 28-Nov-2018 Author: Complete List of Authors: DeBenedictis, Elizabeth; Northwestern University Keten, Sinan; Northwestern University, Mechanical Engineering Page 1 of 10 Please doSoft not Matter adjust margins Soft Matter ARTICLE Mechanical Unfolding of Alpha- and Beta-helical Protein Motifs E. P. DeBenedictis and S. Keten* Received 24th September 2018, Alpha helices and beta sheets are the two most common secondary structure motifs in proteins. Beta-helical structures Accepted 00th January 20xx merge features of the two motifs, containing two or three beta-sheet faces connected by loops or turns in a single protein. Beta-helical structures form the basis of proteins with diverse mechanical functions such as bacterial adhesins, phage cell- DOI: 10.1039/x0xx00000x puncture devices, antifreeze proteins, and extracellular matrices. Alpha helices are commonly found in cellular and extracellular matrix components, whereas beta-helices such as curli fibrils are more common as bacterial and biofilm matrix www.rsc.org/ components. It is currently not known whether it may be advantageous to use one helical motif over the other for different structural and mechanical functions. To better understand the mechanical implications of using different helix motifs in networks, here we use Steered Molecular Dynamics (SMD) simulations to mechanically unfold multiple alpha- and beta- helical proteins at constant velocity at the single molecule scale. We focus on the energy dissipated during unfolding as a means of comparison between proteins and work normalized by protein characteristics (initial and final length, # H-bonds, # residues, etc.). -

Analysis of Spiral Curves in Traditional Cultures
Forum _______________________________________________________________ Forma, 22, 133–139, 2007 Analysis of Spiral Curves in Traditional Cultures Ryuji TAKAKI1 and Nobutaka UEDA2 1Kobe Design University, Nishi-ku, Kobe, Hyogo 651-2196, Japan 2Hiroshima-Gakuin, Nishi-ku, Hiroshima, Hiroshima 733-0875, Japan *E-mail address: [email protected] *E-mail address: [email protected] (Received November 3, 2006; Accepted August 10, 2007) Keywords: Spiral, Curvature, Logarithmic Spiral, Archimedean Spiral, Vortex Abstract. A method is proposed to characterize and classify shapes of plane spirals, and it is applied to some spiral patterns from historical monuments and vortices observed in an experiment. The method is based on a relation between the length variable along a curve and the radius of its local curvature. Examples treated in this paper seem to be classified into four types, i.e. those of the Archimedean spiral, the logarithmic spiral, the elliptic vortex and the hyperbolic spiral. 1. Introduction Spiral patterns are seen in many cultures in the world from ancient ages. They give us a strong impression and remind us of energy of the nature. Human beings have been familiar to natural phenomena and natural objects with spiral motions or spiral shapes, such as swirling water flows, swirling winds, winding stems of vines and winding snakes. It is easy to imagine that powerfulness of these phenomena and objects gave people a motivation to design spiral shapes in monuments, patterns of cloths and crafts after spiral shapes observed in their daily lives. Therefore, it would be reasonable to expect that spiral patterns in different cultures have the same geometrical properties, or at least are classified into a small number of common types. -

Effect of Superhelical Structure on the Secondary Structure of DNA Rings
VOL. 5, PP. 691-696 (1967) Effect of Superhelical Structure on the Secondary Structure of DNA Rings DANIEL GLAUBIGER and JOHN E. HEARST, Departnient of Chemistry, University of California, Berkeley, California Synopsis A quantity, called the linking number, is defined, which specifies the total number of t,wists in a circular helix. The linking number is invariant under continuous deforma- tions of the ring and therefore enables one to calculate the influence of superhelical structures on the secondary helix of a circular molecule. The linking number can be determined by projecting the helix into a plane and counting strand crosses in the projection as described. For example, it has been shown that for each 180" twist in a left-handed superhelix, a right-handed 360" twist is removed from the secondary helix, thus allowing local unwinding. Introduction It is of interest to relate superhelical structure of helical molecules to their secondary structure.' By defining a quantity characteristic of two- stranded circular helical niolecules, related to the helical structure, and in- variant under continuous deformations of the molecule, we are able to evaluate the changes in helical structure brought about by the imposition of superhelical configurations on such molecules. The quantity of interest, called the linking number, is related to the number of turns/cycle for a circular helical molecule.* It will be shown that superhelical structures which are made from helical polymers con- tribute to this number and alter the contribution made by the secondary structure of the molecule to this number. This is made manifest as a change in the number of turns/cycle or as a change in the number of turns/ unit length. -

Influence of the Sequence-Dependent Flexure of DNA on Transcription in E.Coli
Volume 17 Number 22 1989 Nucleic Acids Research Influence of the sequence-dependent flexure of DNA on transcription in E.coli Christina M.Collis, Peter L.Molloy, Gerald W.Both and Horace R.Drew* CSIRO Division of Biotechnology, Laboratory for Molecular Biology, PO Box 184, North Ryde, NSW 2113, Australia Received August 11, 1989; Revised and Accepted October 20, 1989 ABSTRACT In order to study the effects of DNA structure on cellular processes such as transcription, we have made a series of plasmids that locate several different kinds of DNA structure (stiff, flexible or curved) near the sites of cleavage by commonly-used restriction enzymes. One can use these plasmids to place any DNA region of interest (e.g., promoter, operator or enhancer) close to certain kinds of DNA structure that may influence its ability to work in a living cell. In the present example, we have placed a promoter from T7 virus next to the special DNA structures; the T7 promoter is then linked to a gene for a marker protein (chloramphenicol acetyl transferase). When plasmids bearing the T7 promoter are grown in cells of E. coli that contain T7 RNA polymerase, the special DNA structures seem to have little or no influence over the activity of the T7 promoter, contrary to our expectations. Yet when the same plasmids are grown in cells of E. coli that do not contain T7 RNA polymerase, some of the DNA structures show a surprising promoter activity of their own. In particular, the favourable flexibility or curvature of DNA, in the close vicinity of potential -35 and -10 promoter regions, seems to be a significant factor in determining where E.