Effects of White-Nose Syndrome on Bat Diets and Interspecific Competition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forest Health Conditions in Ontario 2013
Some of the information in this document may not be compatible with assistive technologies. If you need any of the information in an alternate format, please contact [email protected]. Forest Health Conditions in Ontario - 2013 Compiled by: • Ontario Ministry of Natural Resources and Forestry, Forests Branch, Forest Health & Silviculture Section and Forest Evaluation and Standards Section, Sault Ste. Marie Ontario and; • Science and Information Branch, Inventory, Monitoring and Assessment Section, Sault Ste. Marie, Ontario; • In cooperation with Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre, Sault Ste. Marie Ontario. © 2015, Queen’s Printer for Ontario Printed in Ontario, Canada Find the Ministry of Natural Resources on‐line at: http://www.ontario.ca For more information on forest health in Ontario visit the Natural Resources website: www.ontario.ca/foresthealth You can also visit the Canadian Forest Service website: www.glfc.cfs.nrcan.gc.ca Cette publication hautement spécialisée Forest Health Conditions in Ontario 2013 n’est disponible qu’en anglais conformément au Règlement 671/92, selon lequel il n’est pas obligatoire de la traduire en vertu de la Loi sur les services en français. Pour obtenir des renseignements en français, veuillez communiquer avec le ministère des Richesses naturelles et des Forêts au [email protected] (705‐945‐6654). ISSN 1913‐617X (Online) ISBN 978‐1‐4606‐7392‐8 (Print) ISBN 978‐1‐4606‐7393‐5 (HTML) Forest tent caterpillar ISBN 978‐1‐4606‐7394‐2 (PDF) Table of Contents Contributors ...................................................................... 4 Provincial Overview: Introduction ...................................................................... 5 Weather patterns ............................................................. 6 Extreme weather and abiotic events .................................6 Insect infestations............................................................ -
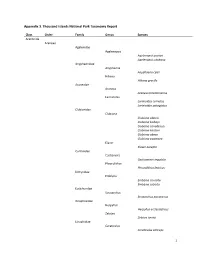
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

African Butterfly News Can Be Downloaded Here
LATE SUMMER EDITION: JANUARY / AFRICAN FEBRUARY 2018 - 1 BUTTERFLY THE LEPIDOPTERISTS’ SOCIETY OF AFRICA NEWS LATEST NEWS Welcome to the first newsletter of 2018! I trust you all have returned safely from your December break (assuming you had one!) and are getting into the swing of 2018? With few exceptions, 2017 was a very poor year butterfly-wise, at least in South Africa. The drought continues to have a very negative impact on our hobby, but here’s hoping that 2018 will be better! Braving the Great Karoo and Noorsveld (Mark Williams) In the first week of November 2017 Jeremy Dobson and I headed off south from Egoli, at the crack of dawn, for the ‘Harde Karoo’. (Is there a ‘Soft Karoo’?) We had a very flexible plan for the six-day trip, not even having booked any overnight accommodation. We figured that finding a place to commune with Uncle Morpheus every night would not be a problem because all the kids were at school. As it turned out we did not have to spend a night trying to kip in the Pajero – my snoring would have driven Jeremy nuts ... Friday 3 November The main purpose of the trip was to survey two quadrants for the Karoo BioGaps Project. One of these was on the farm Lushof, 10 km west of Loxton, and the other was Taaiboschkloof, about 50 km south-east of Loxton. The 1 000 km drive, via Kimberley, to Loxton was accompanied by hot and windy weather. The temperature hit 38 degrees and was 33 when the sun hit the horizon at 6 pm. -

Effects of Fire Frequency on Savanna Butterfly Diversity and Composition: a Preliminary Study
KOEDOE - African Protected Area Conservation and Science ISSN: (Online) 2071-0771, (Print) 0075-6458 Page 1 of 9 Original Research Effects of fire frequency on savanna butterfly diversity and composition: A preliminary study Authors: Fire plays a major role in many biomes, is widely used as a management tool and is likely to be 1,2 Elie Gaget affected by climate change. For effective conservation management, it is essential to understand Catherine L. Parr3,4,5 Clélia Sirami1,6,7 how fire regimes affect different taxa, yet responses of invertebrates are particularly poorly documented. We tested how different fire frequencies influence savanna butterfly diversity Affiliations: and composition by using a long-term savanna fire experiment initiated in 1954 in the Kruger 1 Centre d’Ecologie National Park (South Africa). We compared butterfly abundance, species richness and Fonctionnelle et Evolutive, Montpellier, France community composition across three fire frequencies: high (burnt annually), medium (burnt triennially) and low (burnt twice in 60 years). Plots with high fire frequency hosted higher 2Department of Biology, abundance than medium- or low-frequency plots. Fire frequencies did not affect species University of Turku, Turku, richness, but they led to distinct communities of butterflies. Our findings suggest that, in view Finland of the three fire frequencies tested, a spatial diversity of fire frequencies may increase butterfly diversity at the landscape level in wet savannas. Managers may need to promote a greater 3Department of Earth, Ocean and Ecological Sciences, School diversity of fire frequencies by increasing fire frequency in some areas to provide habitat for of Environmental Sciences, species requiring high fire frequency, and by decreasing fire frequency in a large proportion of University of Liverpool, the landscape to provide fire refuges. -

STATES 00000000A F 24Hr Lawn Insect Killer 32Oz.RTS
92564-17_BioAdvanced SBS 24 hr Lawn Insect Killer_20170928_80_92564_.pdf 24 Hour Lawn Insect Killer READY-TO-SPRAY Mosquitoes KILLS Listed Insects in 24 Hours Ants Fleas KILLS Over * Ticks 500 Insects Chinch Bugs Mata los insectos de la lista en 24 horas Mata más de 500 insectos* Active Ingredients: Mole Crickets Cyfluthrin . 0.75% Other Ingredients . 99.25% Total 100.00% KEEP OUT OF REACH OF CHILDREN See back panel booklet for additional CAUTION precautionary statements *Listed insects NET CONTENTS 32 FL OZ (946ml) OPEN (continuación) Resealable Label VID 24 Hour for Directions & PARA MEJORES RESULTADOS: No regar las hojas dentro de una hora luego de la aplicación. Para evitar daños graves, eliminar las plagas Precautions comienzos de la temporada antes de que se multipliquen. Lawn Insect ® Las personas y las mascotas pueden volver a ingresar al área tratada una vez que se haya secado. Killer READY-TO-SPRAY This product kills listed outdoor pests by contact. Keeping your garden and lawn free of listed pests ALMACENAMIENTO Y ELIMINACIÓN has never been easier. This product is ready to spray. Just attach this bottle to your hose according to ALMACENAMIENTO DEL PESTICIDA directions. Water automatically mixes with the concentrate as you spray. Almacenar en el contenedor original y en un lugar fresco y seco, fuera del alcance de los niños, preferentemente en un armario bajo llave. READ THE LABEL FIRST! Indicaciones MANIPULACIÓN DEL CONTENEDOR en español Si está vacío: • Contenedor no reusable. No volver a rellenar ni utilizar este contenedor. • Colocar el contenedor vacío en la basura o entregarlo para QUICK FACTS después de las reciclar si fuera posible. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -

Pukaskwa Taxonomy Report
Pukaskwa Taxonomy Report Class Order Family Species Arachnida Araneae Agelenidae Agelenopsis utahana Amaurobiidae Callobius bennetti Cybaeopsis euopla Araneidae Hypsosinga rubens Clubionidae Clubiona canadensis Dictynidae Emblyna annulipes Emblyna phylax Linyphiidae Bathyphantes canadensis Ceraticelus atriceps Ceraticelus fissiceps Ceraticelus laetabilis Ceratinopsis nigriceps Dismodicus decemoculatus Drapetisca alteranda Grammonota angusta Lophomma depressum Phlattothrata flagellata Pityohyphantes subarcticus Pocadicnemis americana Sciastes truncatus Scyletria inflata Souessa spinifera Tapinocyba simplex Tapinocyba sp. 1GAB Lycosidae Pardosa hyperborea Pardosa moesta Pardosa xerampelina Philodromidae Philodromus peninsulanus Philodromus rufus vibrans Theridiidae Canalidion montanum Dipoena sp. 1GAB Theridion differens Theridion pictum Thomisidae Xysticus emertoni Xysticus montanensis Mesostigmata Blattisociidae Digamasellidae Dinychidae Laelapidae Parasitidae Phytoseiidae Trematuridae Trichouropoda moseri Pseudoscorpiones Chernetidae Sarcoptiformes Alycidae Ceratozetidae Oribatulidae Scheloribatidae 1 Tegoribatidae Trhypochthoniidae Trhypochthonius cladonicolus Trombidiformes Anisitsiellidae Anystidae Bdellidae Cunaxidae Erythraeidae Eupodidae Hydryphantidae Lebertiidae Limnesiidae Microdispidae Rhagidiidae Scutacaridae Siteroptidae Tetranychidae Trombidiidae Collembola Entomobryomorpha Entomobryidae Entomobrya comparata Entomobrya nivalis Isotomidae Tomoceridae Poduromorpha Brachystomellidae Symphypleona Bourletiellidae Katiannidae -

Forest Health Conditions in Ontario, 2017
Forest Health Conditions in Ontario, 2017 Ministry of Natural Resources and Forestry Forest Health Conditions in Ontario, 2017 Compiled by: • Ontario Ministry of Natural Resources and Forestry, Science and Research Branch © 2018, Queen’s Printer for Ontario Printed in Ontario, Canada Find the Ministry of Natural Resources and Forestry on-line at: <http://www.ontario.ca>. For more information about forest health monitoring in Ontario visit the natural resources website: <http://ontario.ca/page/forest-health-conditions> Some of the information in this document may not be compatible with assistive technologies. If you need any of the information in an alternate format, please contact [email protected]. Cette publication hautement spécialisée Forest Health Conditions in Ontario, 2017 n'est disponible qu'en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec le ministère des Richesses naturelles au <[email protected]>. ISSN 1913-617X (Online) ISBN 978-1-4868-2275-1 (2018, pdf) Contents Contributors ........................................................................................................................ 4 État de santé des forêts 2017 ............................................................................................. 5 Introduction......................................................................................................................... 6 Contributors Weather patterns ................................................................................................... -

Field Instructions for The
FIELD INSTRUCTIONS FOR THE URBAN INVENTORY OF SAN DIEGO, CALIFORNIA 2017 FOREST INVENTORY AND ANALYSIS RESOURCE MONITORING AND ASSESSMENT PROGRAM PACIFIC NORTHWEST RESEARCH STATION USDA FOREST SERVICE Note to User: URBAN FIA Field Guide 7.1 is based on the National CORE Field Guide, Version 7.1. Data elements are national CORE unless indicated as follows: • National CORE data elements that end in “+U” (e.g., x.x+U) have had values,codes, or text added, changed, or adjusted from the CORE program. Any additional URBAN FIA text for a national CORE data element is hi-lighted or shown as an "Urban Note". • All URBAN FIA data elements end in “U” (e.g., x.xU). The text for an URBAN FIA data element is not hi- lighted and does not have a corresponding variable in CORE. • URBAN FIA electronic file notes: • national CORE data elements that are not applicable in URBAN FIA are formatted as light gray or light gray hidden text. • hyperlink cross-references are included for various sections, figures, and tables. *National CORE data elements retain their national CORE field guide data element/variable number but may not retain their national CORE field guide location or sequence within the guide. pg.3 Table of Contents CHAPTER 1 INTRODUCTION . 11 SECTION 1.1 URBAN OVERVIEW. .11 SECTION 1.2 FIELD GUIDE LAYOUT . 12 SECTION 1.3 UNITS OF MEASURE . 12 CHAPTER 2 GENERAL DESCRIPTION . 13 SECTION 2.1 PLOT SETUP . 15 SECTION 2.2 PLOT INTEGRITY . 15 SECTION 2.3 PLOT MONUMENTATION . 15 ITEM 2.3.0.1 MONUMENT TYPE (CORE 0.3.1U) . -

2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory
2010 Season Summary Index NEW WOFTHE~ Zone 1: Yukon Territory ........................................................................................... 3 Alaska ... ........................................ ............................................................... 3 LEPIDOPTERISTS Zone 2: British Columbia .................................................... ........................ ............ 6 Idaho .. ... ....................................... ................................................................ 6 Oregon ........ ... .... ........................ .. .. ............................................................ 10 SOCIETY Volume 53 Supplement Sl Washington ................................................................................................ 14 Zone 3: Arizona ............................................................ .................................... ...... 19 The Lepidopterists' Society is a non-profo California ............... ................................................. .............. .. ................... 2 2 educational and scientific organization. The Nevada ..................................................................... ................................ 28 object of the Society, which was formed in Zone 4: Colorado ................................ ... ............... ... ...... ......................................... 2 9 May 1947 and formally constituted in De Montana .................................................................................................... 51 cember -

Butterflies and Moths of Centre County, Pennsylvania, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail