Material Consideration Radiation Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blending Hydrogen Into Natural Gas Pipeline Networks: a Review of Key Issues
Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues M. W. Melaina, O. Antonia, and M. Penev NREL is a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency & Renewable Energy, operated by the Alliance for Sustainable Energy, LLC. Technical Report NREL/TP-5600-51995 March 2013 Contract No. DE-AC36-08GO28308 Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues M. W. Melaina, O. Antonia, and M. Penev Prepared under Task No. HT12.2010 NREL is a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency & Renewable Energy, operated by the Alliance for Sustainable Energy, LLC. National Renewable Energy Laboratory Technical Report 15013 Denver West Parkway NREL/TP-5600-51995 Golden, Colorado 80401 March 2013 303-275-3000 • www.nrel.gov Contract No. DE-AC36-08GO28308 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. -

Effects of Acute and Chronic Gamma Irradiation on the Cell Biology and Physiology of Rice Plants
plants Article Effects of Acute and Chronic Gamma Irradiation on the Cell Biology and Physiology of Rice Plants Hong-Il Choi 1,† , Sung Min Han 2,†, Yeong Deuk Jo 1 , Min Jeong Hong 1 , Sang Hoon Kim 1 and Jin-Baek Kim 1,* 1 Advanced Radiation Technology Institute, Korea Atomic Energy Research Institute, Jeongeup 56212, Korea; [email protected] (H.-I.C.); [email protected] (Y.D.J.); [email protected] (M.J.H.); [email protected] (S.H.K.) 2 Division of Ecological Safety, National Institute of Ecology, Seocheon 33657, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-63-570-3313 † Both authors contributed equally to this work. Abstract: The response to gamma irradiation varies among plant species and is affected by the total irradiation dose and dose rate. In this study, we examined the immediate and ensuing responses to acute and chronic gamma irradiation in rice (Oryza sativa L.). Rice plants at the tillering stage were exposed to gamma rays for 8 h (acute irradiation) or 10 days (chronic irradiation), with a total irradiation dose of 100, 200, or 300 Gy. Plants exposed to gamma irradiation were then analyzed for DNA damage, oxidative stress indicators including free radical content and lipid peroxidation, radical scavenging, and antioxidant activity. The results showed that all stress indices increased immediately after exposure to both acute and chronic irradiation in a dose-dependent manner, and acute irradiation had a greater effect on plants than chronic irradiation. The photosynthetic efficiency and growth of plants measured at 10, 20, and 30 days post-irradiation decreased in irradiated plants, Citation: Choi, H.-I.; Han, S.M.; Jo, Y.D.; Hong, M.J.; Kim, S.H.; Kim, J.-B. -

Effects of Gamma-Irradiation of Seed Potatoes on Numbers of Stems and Tubers
Netherlands Journal of Agricultural Science 39 (1991) 81-90 Effects of gamma-irradiation of seed potatoes on numbers of stems and tubers A. J. HAVERKORT1, D. I. LANGERAK2 & M. VAN DE WAART1 1 Centre for Agrobiological Research (CABO-DLO), P.O. Box 14, NL 6700 AA Wagenin- gen, Netherlands 2 State Institute for Quality Control of Agricultural Products (RIKILT), P.O. Box 230, NL 6700 AE Wageningen, Netherlands Received 28 november 1990; accepted 8 February 1991 Abstract In field trials with the cultivars Bintje, Jaerla and Spunta, whose seed potatoes were treated with gamma-rays from a ^Co source with doses varying from 0.5 to 27 Gy, tuber yield, har vest index, and number of stems and tubers were determined. A dose of 3 Gy increased the number of tubers by 30 % in Spunta in two out of three trials and by 17 % in one trial in Jaer la, but it did not increase number of tubers in Bintje. Doses of 9 or 10 Gy did not influence the number of tubers nor stems, and decreased harvest index. A dose of 27 Gy yielded off-type plants with reduced yield and number of tubers. Gamma-radiation affected the growth of the apex of the sprout allowing lateral buds or divisions of the affected apex to develop into stems. To achieve larger numbers of tuber-bearing stems, tubers should preferably be irra diated at the start of sprout growth, about 5 months before planting. Keywords: harvest index, gamma irradiation, seed potatoes Introduction Effects of different doses of gamma-rays on potato tubers have been studied exten sively, especially in the 1960s. -

Industrial Applications of Electron Accelerators
Industrial applications of electron accelerators M.R. Cleland Ion Beam Applications, Edgewood, NY 11717, USA Abstract This paper addresses the industrial applications of electron accelerators for modifying the physical, chemical or biological properties of materials and commercial products by treatment with ionizing radiation. Many beneficial effects can be obtained with these methods, which are known as radiation processing. The earliest practical applications occurred during the 1950s, and the business of radiation processing has been expanding since that time. The most prevalent applications are the modification of many different plastic and rubber products and the sterilization of single-use medical devices. Emerging applications are the pasteurization and preservation of foods and the treatment of toxic industrial wastes. Industrial accelerators can now provide electron energies greater than 10 MeV and average beam powers as high as 700 kW. The availability of high-energy, high-power electron beams is stimulating interest in the use of X-rays (bremsstrahlung) as an alternative to gamma rays from radioactive nuclides. 1 Introduction Radiation processing can be defined as the treatment of materials and products with radiation or ionizing energy to change their physical, chemical or biological characteristics, to increase their usefulness and value, or to reduce their impact on the environment. Accelerated electrons, X-rays (bremsstrahlung) emitted by energetic electrons, and gamma rays emitted by radioactive nuclides are suitable energy sources. These are all capable of ejecting atomic electrons, which can then ionize other atoms in a cascade of collisions. So they can produce similar molecular effects. The choice of energy source is usually based on practical considerations, such as absorbed dose, dose uniformity (max/min) ratio, material thickness, density and configuration, processing rate, capital and operating costs. -

Investigation of Electron Beam Irradiated Acrylonitrile-Butadiene-Styrene (Abs) Under Oven Treatment
INVESTIGATION OF ELECTRON BEAM IRRADIATED ACRYLONITRILE-BUTADIENE-STYRENE (ABS) UNDER OVEN TREATMENT JEFFREY CHIN GUO JUN A project report submitted in partial fulfilment of the requirements for the award of Bachelor of Engineering (Hons.) Chemical Engineering Faculty of Engineering and Science Universiti Tunku Abdul Rahman May 2015 ii DECLARATION I hereby declare that this project report is based on my original work except for citations and quotations which have been duly acknowledged. I also declare that it has not been previously and concurrently submitted for any other degree or award at UTAR or other institutions. Signature : Name : JEFFREY CHIN GUO JUN ID No. : 10UEB03372 Date : iii APPROVAL FOR SUBMISSION I certify that this project report entitled “INVESTIGATION OF ELECTRON BEAM IRRADIATED ACRYLONITRILE-BUTADIENE-STYRENE(ABS) UNDER OVEN TREATMENT” was prepared by JEFFREY CHIN GUO JUN has met the required standard for submission in partial fulfilment of the requirements for the award of Bachelor of Engineering (Hons.) Chemical Engineering at Universiti Tunku Abdul Rahman. Approved by, Signature : Supervisor : Prof. Ir. Dr. Tee Tiam Ting Date : iv The copyright of this report belongs to the author under the terms of the copyright Act 1987 as qualified by Intellectual Property Policy of Universiti Tunku Abdul Rahman. Due acknowledgement shall always be made of the use of any material contained in, or derived from, this report. © 2015, Jeffrey Chin Guo Jun. All right reserved. v ACKNOWLEDGEMENTS I would like to thank everyone who had contributed to the successful completion of this project. I would like to express my appreciation to my research supervisor, Prof. -

Bio-9 a Comparison of Y-Irradiation and Microwave Treatments on the Lipids and Microbiological Pattern of Beef Liver
Seventh Conference of Nuclear Sciences & Applications 6-10 February 2000, Cairo, Egypt Bio-9 A comparison of y-irradiation and microwave treatments on the lipids and microbiological pattern of beef liver Farag, R. S .*, Z. Y. Daw2, Farag , S.A 3 and Safaa A. E. ABD El-Wahab3. / Biochemistry Department, Faculty of Agriculture, Cairo University, Giza - Egypt. 2 Microbiology Department. Faculty of Agriculture. Cairo University, Giza - Egypt. 3 Food irradiation Department, National Center for Radiation Research and Technology, Cairo - Egypt. ABSTRACT EG0100123 The effects of y-irradiation treatments (0, 2.5, 5 and, 10 kGy) and microwaves generated from an oven at low and defrost settings for 0.5 , 1 and 2 min on the chemical composition and microbiological aspects of beef liver samples were studied . The chemical and microbiological analyses were performed on the non-treated and treated beef liver immediately after treatments and during frozen storage (-18°C) for 3 months. The chemical analyses of beef liver Hpids showed that acid, peroxide and TBA(Thiobarbituric acid ) values were slightly increased after irradiation treatments and also during frozen storage (-18°C). On the contrary, iodine value of the treated beef liver was decreased. Irradiation treatments remarkably reduced the total bacterial counts in beef liver. The percent reduction of bacterial load for beef liver exposed to microwaves generated from an oven at defrost mode for 2 min and after 3 months at - 18°C was 62% . The bacterial load for beef liver exposed to y-irradiation at 10 kGy after 3 months at - 18°C was decreased by 98%. Hence, y-irradiation treatment was far better than microwave treatment for inhibiting the multiplication of the associated microorganisms with beef liver. -

Beam–Material Interactions
Beam–Material Interactions N.V. Mokhov1 and F. Cerutti2 1Fermilab, Batavia, IL 60510, USA 2CERN, Geneva, Switzerland Abstract This paper is motivated by the growing importance of better understanding of the phenomena and consequences of high-intensity energetic particle beam interactions with accelerator, generic target, and detector components. It reviews the principal physical processes of fast-particle interactions with matter, effects in materials under irradiation, materials response, related to component lifetime and performance, simulation techniques, and methods of mitigating the impact of radiation on the components and environment in challenging current and future applications. Keywords Particle physics simulation; material irradiation effects; accelerator design. 1 Introduction The next generation of medium- and high-energy accelerators for megawatt proton, electron, and heavy- ion beams moves us into a completely new domain of extreme energy deposition density up to 0.1 MJ/g and power density up to 1 TW/g in beam interactions with matter [1, 2]. The consequences of controlled and uncontrolled impacts of such high-intensity beams on components of accelerators, beamlines, target stations, beam collimators and absorbers, detectors, shielding, and the environment can range from minor to catastrophic. Challenges also arise from the increasing complexity of accelerators and experimental set-ups, as well as from design, engineering, and performance constraints. All these factors put unprecedented requirements on the accuracy of particle production predictions, the capability and reliability of the codes used in planning new accelerator facilities and experiments, the design of machine, target, and collimation systems, new materials and technologies, detectors, and radiation shielding and the minimization of radiation impact on the environment. -
![Particle Accelerators and Detectors for Medical Diagnostics and Therapy Arxiv:1601.06820V1 [Physics.Med-Ph] 25 Jan 2016](https://docslib.b-cdn.net/cover/8515/particle-accelerators-and-detectors-for-medical-diagnostics-and-therapy-arxiv-1601-06820v1-physics-med-ph-25-jan-2016-558515.webp)
Particle Accelerators and Detectors for Medical Diagnostics and Therapy Arxiv:1601.06820V1 [Physics.Med-Ph] 25 Jan 2016
Particle Accelerators and Detectors for medical Diagnostics and Therapy Habilitationsschrift zur Erlangung der Venia docendi an der Philosophisch-naturwissenschaftlichen Fakult¨at der Universit¨atBern arXiv:1601.06820v1 [physics.med-ph] 25 Jan 2016 vorgelegt von Dr. Saverio Braccini Laboratorium f¨urHochenenergiephysik L'aspetto pi`uentusiasmante della scienza `eche essa incoraggia l'uomo a insistere nei suoi sogni. Guglielmo Marconi Preface This Habilitation is based on selected publications, which represent my major sci- entific contributions as an experimental physicist to the field of particle accelerators and detectors applied to medical diagnostics and therapy. They are reprinted in Part II of this work to be considered for the Habilitation and they cover original achievements and relevant aspects for the present and future of medical applications of particle physics. The text reported in Part I is aimed at putting my scientific work into its con- text and perspective, to comment on recent developments and, in particular, on my contributions to the advances in accelerators and detectors for cancer hadrontherapy and for the production of radioisotopes. Dr. Saverio Braccini Bern, 25.4.2013 i ii Contents Introduction 1 I 5 1 Particle Accelerators and Detectors applied to Medicine 7 2 Particle Accelerators for medical Diagnostics and Therapy 23 2.1 Linacs and Cyclinacs for Hadrontherapy . 23 2.2 The new Bern Cyclotron Laboratory and its Research Beam Line . 39 3 Particle Detectors for medical Applications of Ion Beams 49 3.1 Segmented Ionization Chambers for Beam Monitoring in Hadrontherapy 49 3.2 Proton Radiography with nuclear Emulsion Films . 62 3.3 A Beam Monitor Detector based on doped Silica Fibres . -

Kocetal Polyoxymethylene 1 1 Business Area
® Engineering Plastic KOCETAL POLYOXYMETHYLENE 1 1 BUSINESS AREA Seoul Ofce (Sales Division) Seoul Office (Sales Division) 1Seoul0th floor Ofce, Kolon (Sales Tow Division)er annex 1-22, Seoul Ofce (Sales Division) Byulyang-Dong, Gwacheon City, 10F, an annex to Kolon Tower 1-22, 10th floor, Kolon Tower annex 1-22, Seoul Ofce (Sales Division) ByulyGyunggi-Do,ang-Dong Korea, Gwacheon City, Byeolyang-dong, Gwacheon City, Gyunggi-Do, Korea Gyunggi-Do, Korea www.kolonplastics.com Headquarters and Plant www.kolonplastics.com Headquarters and Plant Headquarters, Factory and R&D Division Headquarters, Factory and R&D Division 1018, Eungmyeong-dong, Gimcheon-si, 101Headquarters,8, Eungmyung-dong Factory, Gimcand heon-CitR&D Divisiony, Gyeongsangbuk-do, Korea 101GyeongSangBukDo,8, Eungmyung-dong Korea, Gimcheon-City, GyTel:eongSangBukDo, +82-54-420-8491, K 8477orea TFel:ax: +82-54-420-8491, +82-54-420-8369 8477 Fax: +82-54-420-8369 Contact for further information If you want to inquire more detail information on product of KOLONPLASTIC,INC. follow the process written below. 1. Access the Internet hompage of KOLONPLASTIC, INC. www.kolonplastics.com/enghome 2. ‘SALES CONTACT’ category. 3. Then you can see contact number on E-mail, Tel or Fax according to regional groups. About Kolon Plastics Kolon Plastics-Growing with our customers as a POM Global Leader Kolon Plastics was established in March 1996 as a joint venture between Kolon Industries Inc. in Korea and Toray Industries Inc. in Japan. Production began in 1998 with capacity and sales of 25,000MT/year. After the 2nd factory line was completed, we produce 57,000MT of POM and 50,000MT of the other compouding materials a year. -

Why Plastic Products Fail
A Smithers Group Company 0 Why Plastic Products Fail Jenny Cooper – Commercial Manager Dr Chris O’Connor - Director of Consultancy Smithers Rapra Technology Ltd Smithers Rapra 1 To provide our customers with Research, Consultancy and Informat ion on all aspects of Plastics, Rubber & Composite Technology • Smithers Rapra • Polymer Consultancy • Material & Product Testing • Chemical Analysis • Manufacturing & Processing • Research Projects • iSmithers • Training Courses • Polymer Library • Conferences • Publications Smithers Rapra Technology Ltd 1 Polymer Consultancy 2 • Product design & development • Process optimisation • Failure diagnosis • Regulatory advice & testing: • Pharmaceutical & medical devices • Food contact testing Smithers Rapra Technology Ltd The Consequences of Plastic Product Failure 3 • Smithers Rapra has undertaken over 5000 polymer relate product failure investigations • We receive > 25 new plastic cases a week from a diverse clientele: Automotive Defence Aerospace Electronics Agricultural Medical Construction Pharmaceutical Domestic Appliances Offshore Energy Smithers Rapra Technology Ltd 2 Plastic Product Failures on the Rise 4 Product failure is rarely reported. “No one wants their dirty washing aired in public” • Those responsible are naturally generally reluctant to publicise the fact - loss of confidence / credibility in the marketplace • Failure investigation is a covert activity – they can’t be disclosed due to client confidentiality agreements Smithers Rapra Technology Ltd The Consequences of Failure 5 Product -
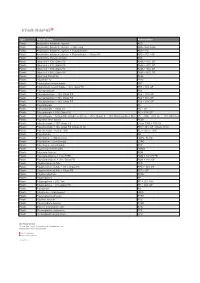
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Present and Future Applications of Industrial Accelerators Craig S
55 Present and Future Applications of Industrial Accelerators Craig S. Nunan Varian Associates, Inc. 56 57 Present and Future Applications of Industrial Accelerators Craig S. Nunan Varian Associates, Inc. Technology transfer - from national laboratories to industry and vice versa implies that concepts, designs, and equipment get transferred. There is also an other mode of transfer which historically has been important - the transfer of ex perienced people. One of the major things that accelerator physics research laboratories have done is to train physicists and engineers in advanced technol ogy. If these people then transfer to industry they can carry with them a level of know-how and creative talent that can be very effective in the on-going applica tion of accelerator-based technology to societal needs. A prime example is Ed Ginzton. Ginzton was a professor of physics at Stanford University and co founder of SLAC, the Stanford Linear Accelerator Center. He was one of the founders of Varian Associates. People from other accelerator laboratories joined Varian over the years. They came from many U.S. and foreign laboratories. Some of the people subsequently left Varian to found other ac celerator companies. A recent example is Bob Hamm, a physicist who trained at Los Alamos Na tional Laboratory, spent two years at Varian, founded AccSys Technology Cor poration, was joined by other people from Los Alamos, and among other things, built the proton linac injector for the proton therapy synchrotron designed and built by Ferrnilab for Lorna Linda University Medical Center. There are other examples of this form of genesis of accelerators in industry.