Checklist of Kansas Turtles and Reptiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bull Snake Class: Reptilia
Pituophis catenifer sayi Bull Snake Class: Reptilia. Order: Squamata. Family: Colubridae. Other names: Gopher Snake, Pine Snake Physical Description: Bull snakes are usually yellow in color, with brown, black or reddish colored blotching or saddle spots on the sides of the snake. There are dark spots placed between the blotches or saddle spots. Below this is a further row of smaller dark spots. The belly is light brown. Many variations in color have been found, including albinos and white variations. This snake has a small head and a large nose shield, which it uses to dig. They often exceed 6 feet in length, with specimens of up to 100 inches being recorded. Males are generally larger then females. The bull snake is a member of the family of harmless snakes, or Colubridae. This is the largest order of snakes, representing two-thirds of all known snake species. Members of this family are found on all continents except Antarctica, widespread from the Arctic Circle to the southern tips of South America and Africa. All but a handful of species are harmless snakes, not having venom or the ability to deliver toxic saliva through fangs. Most harmless snakes subdue their prey through constriction, striking and seizing small rodents, birds or amphibians and quickly wrapping their body around the prey causing suffocation. While other small species such as the common garter snake lack powers to constrict and feed on only small prey it can overpower. Diet in the Wild: Bull snakes eat small mammals, such as mice, rats, large bugs as well as ground nesting birds, lizards and the young of other snakes. -

Life History of the Coppertail Skink (Ctenotus Taeniolatus) in Southeastern Australia
Herpetological Conservation and Biology 15(2):409–415. Submitted: 11 February 2020; Accepted: 19 May 2020; Published: 31 August 2020. LIFE HISTORY OF THE COPPERTAIL SKINK (CTENOTUS TAENIOLATUS) IN SOUTHEASTERN AUSTRALIA DAVID A. PIKE1,2,6, ELIZABETH A. ROZNIK3, JONATHAN K. WEBB4, AND RICHARD SHINE1,5 1School of Biological Sciences A08, University of Sydney, New South Wales 2006, Australia 2Present address: Department of Biology, Rhodes College, Memphis, Tennessee 38112, USA 3Department of Conservation and Research, Memphis Zoo, Memphis, Tennessee 38112, USA 4School of Life Sciences, University of Technology Sydney, Broadway, New South Wales 2007, Australia 5Present address: Department of Biological Sciences, Macquarie University, New South Wales 2109, Australia 6Corresponding author, e-mail: [email protected] Abstract.—The global decline of reptiles is a serious problem, but we still know little about the life histories of most species, making it difficult to predict which species are most vulnerable to environmental change and why they may be vulnerable. Life history can help dictate resilience in the face of decline, and therefore understanding attributes such as sexual size dimorphism, site fidelity, and survival rates are essential. Australia is well-known for its diversity of scincid lizards, but we have little detailed knowledge of the life histories of individual scincid species. To examine the life history of the Coppertail Skink (Ctenotus taeniolatus), which uses scattered surface rocks as shelter, we estimated survival rates, growth rates, and age at maturity during a three-year capture-mark- recapture study. We captured mostly females (> 84%), and of individuals captured more than once, we captured 54.3% at least twice beneath the same rock, and of those, 64% were always beneath the same rock (up to five captures). -

AN INTRODUCTION to Texas Turtles
TEXAS PARKS AND WILDLIFE AN INTRODUCTION TO Texas Turtles Mark Klym An Introduction to Texas Turtles Turtle, tortoise or terrapin? Many people get confused by these terms, often using them interchangeably. Texas has a single species of tortoise, the Texas tortoise (Gopherus berlanderi) and a single species of terrapin, the diamondback terrapin (Malaclemys terrapin). All of the remaining 28 species of the order Testudines found in Texas are called “turtles,” although some like the box turtles (Terrapene spp.) are highly terrestrial others are found only in marine (saltwater) settings. In some countries such as Great Britain or Australia, these terms are very specific and relate to the habit or habitat of the animal; in North America they are denoted using these definitions. Turtle: an aquatic or semi-aquatic animal with webbed feet. Tortoise: a terrestrial animal with clubbed feet, domed shell and generally inhabiting warmer regions. Whatever we call them, these animals are a unique tie to a period of earth’s history all but lost in the living world. Turtles are some of the oldest reptilian species on the earth, virtually unchanged in 200 million years or more! These slow-moving, tooth less, egg-laying creatures date back to the dinosaurs and still retain traits they used An Introduction to Texas Turtles | 1 to survive then. Although many turtles spend most of their lives in water, they are air-breathing animals and must come to the surface to breathe. If they spend all this time in water, why do we see them on logs, rocks and the shoreline so often? Unlike birds and mammals, turtles are ectothermic, or cold- blooded, meaning they rely on the temperature around them to regulate their body temperature. -
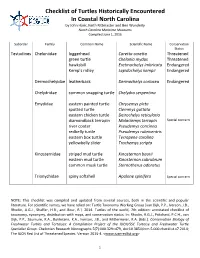
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -

The Importance of Assessing Climate Change Vulnerability to Address Species Conservation Karen E
Issues and Perspectives The Importance of Assessing Climate Change Vulnerability to Address Species Conservation Karen E. Bagne,* Megan M. Friggens, Sharon J. Coe, Deborah M. Finch U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station, Albuquerque, New Mexico 87102 Present address of K.E. Bagne: Department of Biology, Kenyon College, Gambier, Ohio 43022 Abstract Species conservation often prioritizes attention on a small subset of ‘‘special status’’ species at high risk of extinction, but actions based on current lists of special status species may not effectively moderate biodiversity loss if climate change alters threats. Assessments of climate change vulnerability may provide a method to enhance identification of species at risk of extinction. We compared climate change vulnerability and lists of special status species to examine the adequacy of current lists to represent species at risk of extinction in the coming decades. The comparison was made for terrestrial vertebrates in a regionally important management area of the southwestern United States. Many species not listed as special status were vulnerable to increased extinction risk with climate change. Overall, 74% of vulnerable species were not included in lists of special status and omissions were greatest for birds and reptiles. Most special status species were identified as additionally vulnerable to climate change impacts and there was little evidence to indicate the outlook for these species might improve with climate change, which suggests that existing conservation efforts will need to be intensified. Current special status lists encompassed climate change vulnerability best if climate change was expected to exacerbate current threats, such as the loss of wetlands, but often overlooked climate-driven threats, such as exceeding physiological thresholds. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

TPWD White List
TPWD White List Frogs and Toads Great Plains toad (Bufo cognatus) Green toad (Bufo debilis) Red-spotted toad (Bufo punctatus) Texas toad (Bufo speciosus) Gulf Coast toad (Bufo valliceps) Woodhouse’s toad (Bufo woodhousei) Green treefrog (Hyla cinerea) Bull frog (Rana catesbeiana) Couch’s spadefoot (Scaphiopus couchii) Plains spadefoot (Spea bombifrons) New Mexico spadefoot (Spea multiplicata) Salamanders Tiger salamander (Ambystoma tigrinum) Lizards Green anole (Anolis carolinensis) Chihuahuan spotted whiptail (Aspidoscelis exsanguis) Texas spotted whiptail (Aspidoscelis gularis) Marbled whiptail (Aspidoscelis marmoratus) Six-lined racerunner (Aspidoscelis sexlineatus) Checkered whiptail (Aspidoscelis tesselatus) Texas banded gecko (Coleonyx brevis) Greater earless lizard (Cophosaurus texanus) Collared lizard (Crotaphytus collaris) Five-lined skink (Eumeces fasciatus) Great plains skink (Eumeces obsoletus) Texas alligator lizard (Gerrhonotus infernalis) Lesser earless lizard (Holbrookia maculata) Crevice spiny lizard (Sceloporus poinsettii) Prairie lizard (Sceloporus undulatus) Ground skink (Scincella lateralis) Tree lizard (Urosaurus ornatus) Side-blotched lizard (Uta stansburiana) Snakes Copperhead (Agkistrodon contortrix) Cottonmouth (Agkistrodon piscivorus) Glossy snake (Arizona elegans) Trans-Pecos rat snake (Bogertophis subocularis) Racer (Coluber constrictor) Western diamondback rattlesnake (Crotalus atrox) Rock rattlesnake (Crotalus lepidus) Blacktail rattlesnake (Crotalus molossus) Mojave rattlesnake (Crotalus scutulatus) Prairie -

Report of Two Subspecies of an Alien Turtle, Trachemys Scripta Scripta and Trachemys Scripta Elegans (Testudines: Emydidae) Shar
Correspondence ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.ecol-mne.com Report of two subspecies of an alien turtle, Trachemys scripta scripta and Trachemys scripta elegans (Testudines: Emydidae) sharing the same habitat on the island of Zakynthos, Greece ALEKSANDAR UROŠEVI Ć University of Belgrade, Institute for Biological Research “Siniša Stankovi ć”, Bulevar despota Stefana 142, 11000 Belgrade, Serbia, E-mail: [email protected] Received 11 December 2014 │ Accepted 26 December 2014 │ Published online 28 December 2014. Introduction of alien aquatic turtles, especially the invasive red-eared slider, Trachemys scripta elegans (Wied-Neuwied 1839) has been noted as a global problem (Scalera 2006; Bringsøe 2006). In Greece only, introductions of the red-eared slider have already been published for several localities: Athens, Corfu, Crete, Kos and Zakynthos (Bruekers 1993; Bruekers et al . 2006; Zenetos et al . 2009). Until the EU banned the import and trade of the red-eared slider (Council Regulation No. 338/97), tens of millions of these turtles had been imported into Europe (Bringsøe 2006). Since the ban, the yellow-bellied slider Trachemys scripta scripta (Schoepff 1792) emerged in the European pet trade as one of the “substitute” species and subspecies (Adrados et al. 2002; Bringsøe 2006). Although it is sold in smaller quantities, and at a higher price, numbers of this subspecies found in the wild in Europe are increasing (Bringsøe 2006). The spread of T. s. scripta has been documented in Spain (Martínez Silvestre et al . 2006; Alarcos et al . 2010; Valdeón et al . 2010), Sweden, Finland (Bringsøe 2006) and Austria (Kleewein 2014). -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

ISSN 2330-6025 SWCHR BULLETIN Published Quarterly by the SOUTHWESTERN CENTER for HERPETOLOGICAL RESEARCH (SWCHR) P.O
ISSN 2330-6025 SWCHR BULLETIN Published Quarterly by THE SOUTHWESTERN CENTER FOR HERPETOLOGICAL RESEARCH (SWCHR) P.O. Box 624 Seguin TX 78156 www.southwesternherp.com ISSN 2330-6025 OFFICERS 2010-2012 COMMITTEE CHAIRS PRESIDENT COMMITTEE ON COMMON AND Tom Lott SCIENTIFIC NAMES Tom Lott VICE PRESIDENT Todd Hughes RANGE MAP COMMITTEE Tom Lott SECRETARY AWARDS AND GRANTS COMMITTEE Diego Ortiz (vacant) EXECUTIVE DIRECTOR COMMUNICATIONS COMMITTEE Gerald Keown Gerald Keown BOARD MEMBERS ACTIVITIES AND EVENTS COMMITTEE Toby Brock Diego Ortiz Riley Campbell NOMINATIONS COMMITTEE Hans Koenig Gerald Keown EDITORIAL STAFF EDUCATION COMMITTEE (vacant) EDITOR Chris McMartin MEMBERSHIP COMMITTEE Toby Brock TECHNICAL EDITOR Linda Butler CONSERVATION COMMITTEE (vacant) ABOUT SWCHR Originally founded by Gerald Keown in 2007, SWCHR is a Texas non-profit association created under the provisions of the Texas Uniform Unincorporated Non-Profit Association Act, Chapter 252 of the Texas Business Organizations Code, governed by a board of directors and dedicated to promoting education of the Association’s members and the general public relating to the natural history, biology, taxonomy, conservation and preservation needs, field studies, and captive propagation of the herpetofauna indigenous to the American Southwest. SWCHR BULLETIN 1 Fall 2011 TABLE OF CONTENTS A Message from the President, Tom Lott . 2 Upcoming Event: Sanderson Snake Days . 3 Summary of July 17, 2011 Board Meeting, Gerald Keown . 4 TPWD Reptile & Amphibian Stamp FAQ, Scott Vaca . .6 Amended Texas Parks and Wildlife Code Pertaining to Reptiles and Amphibians, with Commentary by Dr. Andy Gluesenkamp. 8 An Annotated Checklist of the Snakes of Atascosa County, Texas, Tom Lott . 10 2011 Third Quarter Photographs of the Month . -
Common Species
Common Species on District Lands The Southwest Florida Water Management District (District) manages water resources in the west- central portion of Florida according to legislative mandates. The District uses public land acquisition funds to protect key lands for water resources. The natural communities, plants and animals on these lands also benefi t from a level of protection that can be experienced and enjoyed through recreational opportunities made available on District lands. Every year, approximately 2.5 million people visit the more than 436,000 acres of public conservation lands acquired by the District and its partners. The lands are open to the public for activities such as hiking, bicycling, hunting, horseback riding, fi shing, camping, picnicking and studying nature. The District provides these recreational opportunities so that residents may experience healthy natural communities. An array of natural landscapes is available from the meandering Outstanding Florida Southwest Florida Water Management District Waters riverine systems, the cypress domes and strands, swamps and hammocks, to the scrub, sandhill, and pine fl atwoods. The Common Species on District Lands brochure provides insight to what you may experience while recreating on public lands managed by the District. CONSERVATION LANDS Southwest Florida Water Management District Mammals Southwest Florida Water Management District iStock W Barbour - Smithsonian Institute Roger Virginia Brazilian Opossum Free-Tailed Bat Didelphis virginiana Tadarida brasiliensis iStock Ivy