Unidirectional Rotation in a Mechanically Interlocked Molecular Rotor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Academy Elects IMS Fellows Have You Voted Yet?
Volume 38 • Issue 5 IMS Bulletin June 2009 National Academy elects IMS Fellows CONTENTS The United States National Academy of Sciences has elected 72 new members and 1 National Academy elects 18 foreign associates from 15 countries in recognition of their distinguished and Raftery, Wong continuing achievements in original research. Among those elected are two IMS Adrian Raftery 2 Members’ News: Jianqing Fellows: , Blumstein-Jordan Professor of Statistics and Sociology, Center Fan; SIAM Fellows for Statistics and the Social Sciences, University of Washington, Seattle, and Wing Hung Wong, Professor 3 Laha Award recipients of Statistics and Professor of Health Research and Policy, 4 COPSS Fisher Lecturer: Department of Statistics, Stanford University, California. Noel Cressie The election was held April 28, during the business 5 Members’ Discoveries: session of the 146th annual meeting of the Academy. Nicolai Meinshausen Those elected bring the total number of active members 6 Medallion Lecture: Tony Cai to 2,150. Foreign associates are non-voting members of the Academy, with citizenship outside the United States. Meeting report: SSP Above: Adrian Raftery 7 This year’s election brings the total number of foreign 8 New IMS Fellows Below: Wing H. Wong associates to 404. The National Academy of Sciences is a private 10 Obituaries: Keith Worsley; I.J. Good organization of scientists and engineers dedicated to the furtherance of science and its use for general welfare. 12-3 JSM program highlights; It was established in 1863 by a congressional act of IMS sessions at JSM incorporation signed by Abraham Lincoln that calls on 14-5 JSM tours; More things to the Academy to act as an official adviser to the federal do in DC government, upon request, in any matter of science or 16 Accepting rejections technology. -

Stereochemistry, Alkyl Halide Substitution (SN1 & SN2)
Chem 261 Assignment & Lecture Outline 3: Stereochemistry, Alkyl Halide Substitution (SN1 & SN2) Read Organic Chemistry, Solomons, Fryle & Snyder 12th Edition (Electronic) • Functional Group List – Learn to recognize – Please see Green Handout – also p 76 of text • Periodic Table – Inside Front Cover - know 1st 10 elements (up through Neon) • Relative Strength of Acids and Bases – Inside Front cover (reference only) • Chapter 5 – Stereochemistry • Chapter 6 –Ionic Reactions: Nucleophilic Substitution of Alkyl Halides Problems: Do Not turn in, answers available in "Study Guide Student Solutions Manual " Solomons, Fryle, Snyder • Chapter 5: 5.1 to 5.15; 5.18 to 5.21; 5.26; 5.28; 5.33a-d; 5.46 • Chapter 6: 6.1 to 6.5; 6.7 to 6.10; 6.13; 6.20; 6.26; 6.27 Lecture Outline # 3 I. Comparison of 2 Structures: Same Molecular Formula ? -> If Yes, Possibly Isomers or Identical Same Arrangement (Sequence) of Groups ? If No -> Structural Isomers If Yes -> Superposable? If Yes -> Identical Structures If No -> Stereoisomers Non-Superposable Mirror Images ? If NO -> Diastereomers If Yes -> Enantiomers II. Chirality and Stereoisomers A. The Concept of Chirality 1. Identification of chiral objects a) achiral = not chiral b) planes of symmetry within a molecule 2. Types of stereoisomers – enantiomers and diastereomers B. Location of stereogenic (chiral) centres – 4 different groups on tetrahedral atom 1. Enantiomers & diastereomers 2. Meso compounds - chiral centers with plane of symmetry within molecule 3. Molecules with more than one chiral centre 4. Recognition of chiral centers in complex molecules - cholesterol - 8 chiral centres Drawing the enantiomer of cholesterol and its potential 255 stereoisomers 5. -

Probing the Dynamics of the Imine-Based Pentafoil Knot and Pentameric Circular Helicate Assembly † ‡ ‡ § ‡ † ‡ Jean-Francoiş Ayme, , Jonathon E
This is an open access article published under a Creative Commons Attribution (CC-BY) License, which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. Article Cite This: J. Am. Chem. Soc. 2019, 141, 3605−3612 pubs.acs.org/JACS Probing the Dynamics of the Imine-Based Pentafoil Knot and Pentameric Circular Helicate Assembly † ‡ ‡ § ‡ † ‡ Jean-Francoiş Ayme, , Jonathon E. Beves, , Christopher J. Campbell, and David A. Leigh*, , † School of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, United Kingdom ‡ School of Chemistry, University of Edinburgh, The King’s Buildings, West Mains Road, Edinburgh EH9 3JJ, United Kingdom *S Supporting Information ABSTRACT: We investigate the self-assembly dynamics of an imine-based pentafoil knot and related pentameric circular helicates, each derived from a common bis(formylpyridine)- bipyridyl building block, iron(II) chloride, and either monoamines or a diamine. The mixing of circular helicates derived from different amines led to the complete exchange of the N-alkyl residues on the periphery of the metallo-supramolecular scaffolds over 4 days in DMSO at 60 °C. Under similar conditions, deuterium-labeled and nonlabeled building blocks showed full dialdehyde building block exchange over 13 days for open circular helicates but was much slower for the analogous closed-loop pentafoil knot (>60 days). Although both knots and open circular helicates self-assemble under thermodynamic control given sufficiently long reaction times, this is significantly longer than the time taken to afford the maximum product yield (2 days). Highly effective error correction occurs during the synthesis of imine-based pentafoil molecular knots and pentameric circular helicates despite, in practice, the systems not operating under full thermodynamic control. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

Weak Functional Group Interactions Revealed Through Metal-Free Active Template Rotaxane Synthesis
ARTICLE https://doi.org/10.1038/s41467-020-14576-7 OPEN Weak functional group interactions revealed through metal-free active template rotaxane synthesis Chong Tian 1,2, Stephen D.P. Fielden 1,2, George F.S. Whitehead 1, Iñigo J. Vitorica-Yrezabal1 & David A. Leigh 1* 1234567890():,; Modest functional group interactions can play important roles in molecular recognition, catalysis and self-assembly. However, weakly associated binding motifs are often difficult to characterize. Here, we report on the metal-free active template synthesis of [2]rotaxanes in one step, up to 95% yield and >100:1 rotaxane:axle selectivity, from primary amines, crown ethers and a range of C=O, C=S, S(=O)2 and P=O electrophiles. In addition to being a simple and effective route to a broad range of rotaxanes, the strategy enables 1:1 interactions of crown ethers with various functional groups to be characterized in solution and the solid state, several of which are too weak — or are disfavored compared to other binding modes — to be observed in typical host–guest complexes. The approach may be broadly applicable to the kinetic stabilization and characterization of other weak functional group interactions. 1 Department of Chemistry, University of Manchester, Manchester M13 9PL, UK. 2These authors contributed equally: Chong Tian, Stephen D. P. Fielden. *email: [email protected] NATURE COMMUNICATIONS | (2020) 11:744 | https://doi.org/10.1038/s41467-020-14576-7 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-14576-7 he bulky axle end-groups of rotaxanes mechanically lock To explore the scope of this unexpected method of rotaxane Trings onto threads, preventing the dissociation of the synthesis, here we carry out a study of the reaction with a series of components even if the interactions between them are not related electrophiles. -

Volume 70, No. 3, October 2006
Inside Volume 70, No.3, October 2006 Articles and Features 70 Structure Determination of New Algal Toxins using NMR Methods Alistair L. Wilkins*,a and Christopher O. Milesb,c 74 Faecal Sterols and Fluorescent Whiteners as Indicators of the Source of Faecal Contamination Megan Devane,* Darren Saunders and Brent Gilpin 78 The Curious Case of Phosphate Solubility Andrew J. Pratt 82 Water Oxidation by Ruthenium Dimers Matthew I. J. Polson 85 Fluorinated Analogues of Biological Molecules: Accessing New Chemical, Physical and Biological Properties Michael Edmondsa and Victoria Peddieb 88 Drug Discovery in the New Millennium Steven G. Aitken 93 2006 NZIC Salary Survey Summary 98 Chemical Processes In New Zealand – Where To From Here? 101 38th International Chemistry Olympiad Regular Columns 99 Patent Proze 102 NZIC News 108 Conference Calendar Advertisers Index Cover Shimadzu Scientific Instruments Ltd Back Cover Shimadzu Scientific Instruments Ltd Inside Front Cover Global Science Inside Back Cover Pacific Laboratory Products 81 NZIC Conference 84 Graham B Jackson: Certified Reference Materials Disclaimer The New Zealand Institute of Scientific Editor The views and opinions expressed in Chemistry Incorporated Professor B. Halton, FRSNZ, Hon, FNZIC Chemistry in New Zealand are those of the PO Box 39-112 School of Chemical and Physical Sciences individual authors and are not necessarily those Harewood, Christchurch Victoria University of the publisher, the Editorial Board or the PO Box 600 Phone: +64 3 359 7275 New Zealand Institute of Chemistry. Whilst Wellington, New Zealand Fax: +64 3 359 7248 the publisher has taken every precaution to Email: NZIC.Offi[email protected] Phone: +64 4 463 5954 ensure the total accuracy of material contained Fax: +64 4 463 5241 in Chemistry in New Zealand, no responsibility Managing Editors and Publishers Email: [email protected] for errors or omissions will be accepted. -

Alkyl Halides
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp3 orbital of an alkyl group. CHCl3 (Chloroform: organic solvent) CF2Cl2 (Freon-12: refrigerant CFC) CF3CHClBr (Halothane: anesthetic) Halogen atoms are more electronegative than carbon atoms, and so the C-Hal bond is polarized. The C-Hal (often written C-X) bond is polarized in such a way that there is partial positive charge on the carbon and partial negative charge on the halogen. Ch06 Alkyl Halides (landscape).docx Page 1 Dipole moment Electronegativities decrease in the order of: F > Cl > Br > I Carbon-halogen bond lengths increase in the order of: C-F < C-Cl < C-Br < C-I Bond Dipole Moments decrease in the order of: C-Cl > C-F > C-Br > C-I = 1.56D 1.51D 1.48D 1.29D Typically the chemistry of alkyl halides is dominated by this effect, and usually results in the C-X bond being broken (either in a substitution or elimination process). This reactivity makes alkyl halides useful chemical reagents. Ch06 Alkyl Halides (landscape).docx Page 2 Nomenclature According to IUPAC, alkyl halides are treated as alkanes with a halogen (Halo-) substituent. The halogen prefixes are Fluoro-, Chloro-, Bromo- and Iodo-. Examples: Often compounds of CH2X2 type are called methylene halides. (CH2Cl2 is methylene chloride). CHX3 type compounds are called haloforms. (CHI3 is iodoform). CX4 type compounds are called carbon tetrahalides. (CF4 is carbon tetrafluoride). Alkyl halides can be primary (1°), secondary (2°) or tertiary (3°). Other types: A geminal (gem) dihalide has two halogens on the same carbon. -

The Chemistry of Tertiary Amides and Related Compounds
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 The heC mistry of Tertiary Amides and Related Compounds. Kalil Phillip Ieyoub Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ieyoub, Kalil Phillip, "The heC mistry of Tertiary Amides and Related Compounds." (1967). LSU Historical Dissertations and Theses. 1252. https://digitalcommons.lsu.edu/gradschool_disstheses/1252 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-8783 IEYOUB, Kalil Phillip, 1935- THE CHEMISTRY OF TERTIARY AMIDES AND RELATED COMPOUNDS. Louisiana State University and Agricultural and Mechanical College, Ph.D., 1967 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. THE CHEMISTRY OE TERTIARY AMIDES AND RELATED COMPOUNDS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by K alil P h illip leyoub B.S., McNe.ese State College, 195^ M.S., Louisiana State University, I 965 January, 1967 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ACKNOWLEDGMENT The author wishes to express his sincere appreciation for the guidance given him by Dr. -

II. Nomenclature Rules for Alkenes 1. the Parent Name Will Be the Longest
1 Lecture 9 II. Nomenclature Rules For Alkenes 1. The parent name will be the longest carbon chain that contains both carbons of the double bond. Drop the -ane suffix of the alkane name and add the –ene suffix. Never name the double bond as a prefix. If a double bond is present, you have an alkene, not an alkane. alkane + -ene = alkene 2. Begin numbering the chain at the end nearest the double bond. Always number through the double bond and identify its position in the longest chain with the lower number. In the older IUPAC rules the number for the double bond was placed in front of the stem name with a hyphen. Under the newer rules, the number for the double bond is placed right in front of “ene”, with hyphens. We will use the newer rules for specifying the location of pi bonds. 1 2 3456 H3CCHCH CH 2 CH2 CH3 hex-2-ene (newer rules) 2-hexene (older rules) 3. Indicate the position of any substituent group by the number of the carbon atom in the parent (longest) chain to which it is attached. CH 1 2 345 3 H3CCHCHCHCH2 CH CH3 6 7 CH3 Numbering is determined by the double bond, not the branches, because the double bond has 5,6-dimethylhept-3-ene (newer rules) higher priority than any alkyl branch. 5,6-dimethyl-3-heptene (older rules) 4. Number cycloalkenes so that the double bond is 1,2 (number through the double bond). Number in the direction about the ring so that the lowest number is used at the first point of difference. -
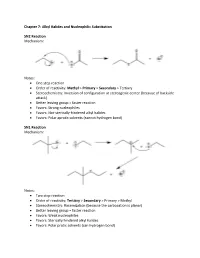
Alkyl Halides and Nucleophilic Substitution SN2 Reaction
Chapter 7: Alkyl Halides and Nucleophilic Substitution SN2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Methyl > Primary > Secondary > Tertiary • Stereochemistry: Inversion of configuration at stereogenic center (because of backside attack) • Better leaving group = faster reaction • Favors: Strong nucleophiles • Favors: Not-sterically-hindered alkyl halides • Favors: Polar aprotic solvents (cannot hydrogen bond) SN1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary > Methyl • Stereochemistry: Racemization (because the carbocation is planar) • Better leaving group = faster reaction • Favors: Weak nucleophiles • Favors: Sterically hindered alkyl halides • Favors: Polar protic solvents (can hydrogen bond) Important Trends Chapter 8: Alkyl Halides and Elimination Reactions E2 Reaction Mechanism: Notes: • One step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: antiperiplanar arrangement of H and X • Better leaving group = faster reaction • Favors: Polar aprotic solvents, strong bases • Products follow Zaitsev rule (more substituted alkene is the major product) E1 Reaction Mechanism: Notes: • Two step reaction • Order of reactivity: Tertiary > Secondary > Primary • Stereochemistry: Trigonal planar carbocation intermediate • Better leaving group = faster reaction • Favors: Polar protic solvents, weak bases • Products follow Zaitsev rule Chapter 9: Alcohols, Ethers, and Epoxides Preparation of Alcohols Mechanism: Notes: • SN2 mechanism -

Top-25 Publications
David Leigh (University of Manchester, UK) – 25 most significant publications (reverse chronological order) 1. "A catalysis-driven artificial molecular pump", S. Amano, S. D. P. Fielden and D. A. Leigh, Nature 594, 529-534 (2021). 2. "A molecular endless (74) knot", D. A. Leigh, J. J. Danon, S. D. P. Fielden, J.-F. Lemonnier, G. F. S. Whitehead and S. L. Woltering, Nat. Chem. 13, 117-122 (2021). 3. "Self-assembly of a layered 2D molecularly woven fabric", D. P. August, R. A. W. Dryfe, S. J. Haigh, P. R. C. Kent, D. A. Leigh, J.-F. Lemonnier, Z. Li, C. A. Muryn, L. I. Palmer, Y. Song, G. F. S. Whitehead and R. J. Young, Nature 588, 429-435 (2020). 4. "Tying different knots in a molecular strand", D. A. Leigh, F. Schaufelberger, L. Pirvu, J. Halldin Stenlid, D. P. August and J. Segard, Nature 584, 562-568 (2020). 5. "Rotary and linear molecular motors driven by pulses of a chemical fuel", S. Erbas-Cakmak, S. D. P. Fielden, U. Karaca, D. A. Leigh, C. T. McTernan, D. J. Tetlow and M. R. Wilson, Science 358, 340-343 (2017). Cited 154 times as of 23.6.21 (GooSch). 6. "Stereodivergent synthesis with a programmable molecular machine", S. Kassem, A. T. L. Lee, D. A. Leigh, V. Marcos, L. I. Palmer and S. Pisano, Nature 549, 374-378 (2017). Cited 113 times as of 23.6.21 (GooSch). 7. "Braiding a molecular knot with eight crossings", J. J. Danon, A. Krüger, D. A. Leigh, J.-F. Lemonnier, A. J. Stephens, I. -

The Magic of Chemistry David Leigh Has a Love of Chemistry...And Magic
Chemical Science Interview The magic of chemistry David Leigh has a love of chemistry...and magic. Alison Stoddart finds out more Who inspired you to become a scientist? You like to practice magic. What is the most magical My high school chemistry teacher Dave Clarke. I thing about chemistry to you? think that’s a common thing amongst chemists. Chemistry allows you create things that never He made chemistry seem fun and exciting. There were and to change the world and change society, were several others from my class who went on and that’s an amazing thing. James Clerk Maxwell, to do chemistry. Barry Moore, on the chemistry the 19th century Scottish physicist, came up with faculty at Strathclyde, also went to my high school. the theory that light was really electromagnetic radiation. A feat of which Richard Feynman said, What motivated you to study molecular machines? ‘…ten thousand years from now - there can be little I worked in Fraser Stoddart’s group before he doubt that the most significant event of the 19th made any catenanes or rotaxanes. We made our century will be judged as Maxwell’s discovery of first catenane about five years after Fraser did. I the laws of electrodynamics.’ I think there are still could see that there was nothing interesting about those kinds of things to be discovered and there is a catenane or a rotaxane in itself. The interesting still that impact to be had on society. thing about them is the possibility to control a well-defined, large-amplitude motion. That’s How long have you been a magician? what you need to make a molecular machine.